Clinical trials are at the very foundation of medical research and supply vital proof that new treatments are both safe and effective.
While robust, the rigidity of conventional clinical trials often prevents modification once started. However, adaptive clinical trials in the last few years represent a more dynamic and efficient approach that could help to streamline the drug development process and generate better treatment outcomes for the patients.
What Are Adaptive Clinical Trials?
Adaptive clinical trials are those in which modifications or adjustments to aspects of trial procedures may be made within a trial after an interim data analysis. Such changes could range from the sample size, dosing regimen, or even the patient population under study. The hallmark of adaptive trials is flexibility; they are structured so that they may evolve with the accumulating data without losing the integrity or even the validity of the study.
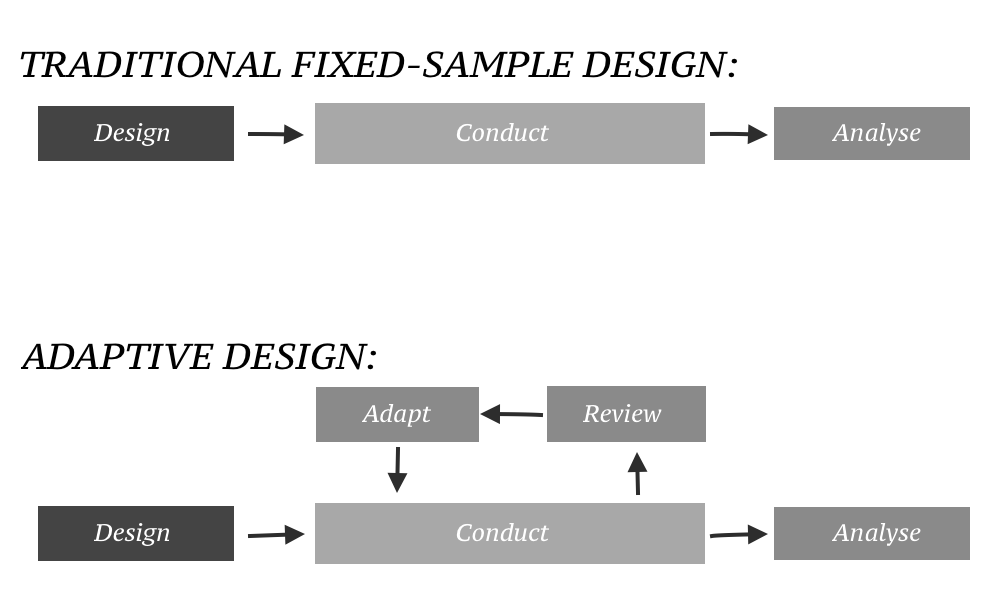
Rationale for Adaptive Trials
The classical fixed design of clinical trials, although methodologically sound, can be an inefficient and slow way. Fixed trials do not allow for such adjustments once a trial has started, so if initial assumptions are wrong or early results show the need to change direction, then the remainder of a trial may proceed sub-optimally.
This can be a waste of resources and time that could have been saved and ultimately misses the chance to hit on effective treatments earlier. Adaptive trials allow real-time adjustments based on pre-specified rules and interim data analyses. This would further have the following benefits:
- Improved efficiency: Resources can be allocated more efficiently, probably with reduced overall time and cost of conducting the trial.
- Ethics: Patients receive fewer inadequate dosages and are assigned to treatment arms that are not effective—this is because the prosecution can adapt in favor of more promising interventions.
- Flexibility: Trials can adapt to emerging information regarding new scientific discoveries or changing standards of care.
Types of Adaptations in Clinical Trials
There are a variety of adaptations possible in an adaptive clinical trial. Some common types include:
- Sample Size Re-estimation: If interim data indicate that the initial sample size is insufficient to achieve the desired power of the study, then the sample size can be increased.
- Adaptive Randomization: Includes changes in the probability of assigning a patient to various treatment arms based on interim results—more patients assigned to the more promising treatments.
- Group Sequential Designs: Permit the stopping of the trial for efficacy, futility, or safety based on the results of an interim analysis.
- Dose Finding: Dose-finding adaptive studies, such as the continual reassessment method, adapt the dose levels being tested based on patients' responses to treatment, to ascertain rapidly the optimal dose.
- Biomarker-Adaptive Designs: These trials would adapt based on a patient's biomarker status, offering an avenue for some truly personalized approaches to treatment.
Statistical Considerations and Challenges
Those topics naturally lead to complex statistical challenges since these kinds of trials differ from conventional trials by design and conduct due to their very nature of being adaptive. The trial design must include pre-specified adaptation rules and, considering the multiplicity of analyses to control the type I error, that is, false positives.
To this end, statistical solid methods and a careful planning process are essential to ensure the validity and integrity of the trial. Adaptive trials drive sophisticated simulation studies at the planning stage to anticipate how different adaptive scenarios will lead to the outcomes of the trial. Regulatory bodies, that is, the FDA and EMA, even issue guidelines on the design and implementation of adaptive trials to ensure that the result obtained is valid and trustworthy.
Regulatory and Ethical Concerns
Regulators have slowly accepted the potential of adaptive designs and have developed strategies to facilitate their use. The FDA's Critical Path Initiative, as well as guidelines initiated by the European Medicines Agency (EMA) on adaptive clinical trials, establish routes for the approval and conduct of these innovative designs.
From an ethical perspective, adaptive trials may find greater acceptance since many require fewer patients to achieve valid results and may entail fewer patients being exposed to treatments that later prove to be without value. Of course, transparency and the maintenance of patient trust remain paramount. Informed consent documents should clearly describe the adaptive nature of the trial and any potential effects on participants.
Future Directions
Promising the continuous advancement in statistical methodologies, computational power, and regulatory framework practically sets the future of adaptive clinical trials. Overcoming most of the challenges that this approach has in developing targeted therapies, it is quite suitable in the field of precision medicine.
Furthermore, its adaptive trial model can be augmented by adding real-world data and digital health technologies. Results from electronic health records and wearable devices, among other sources, offer a pathway for real-time feedback so that designs of trials could be all the more dynamic and responsive.
Conclusion
Adaptive clinical trials constitute a significant advancement in the field of clinical investigation. They offer an approach to testing new treatments that is more efficient, ethical, and flexible because modifications are permitted upon interim data. They pose specific challenges, particularly in statistical and regulatory considerations.
Still, the advantages brought about in terms of speed, cost, and patient outcomes will make them a desirable option for clinical trials in the future. With adaptive trial design, moving ahead on new frontiers where no man has gone before in medical research, these forms of clinical trials are becoming much more critical to ensure new treatments for improved health and well-being for patients worldwide.
Want to know more about how Octalsoft’s eClinical software suite can help you expedite the effectiveness and efficiency of your next clinical trial?



