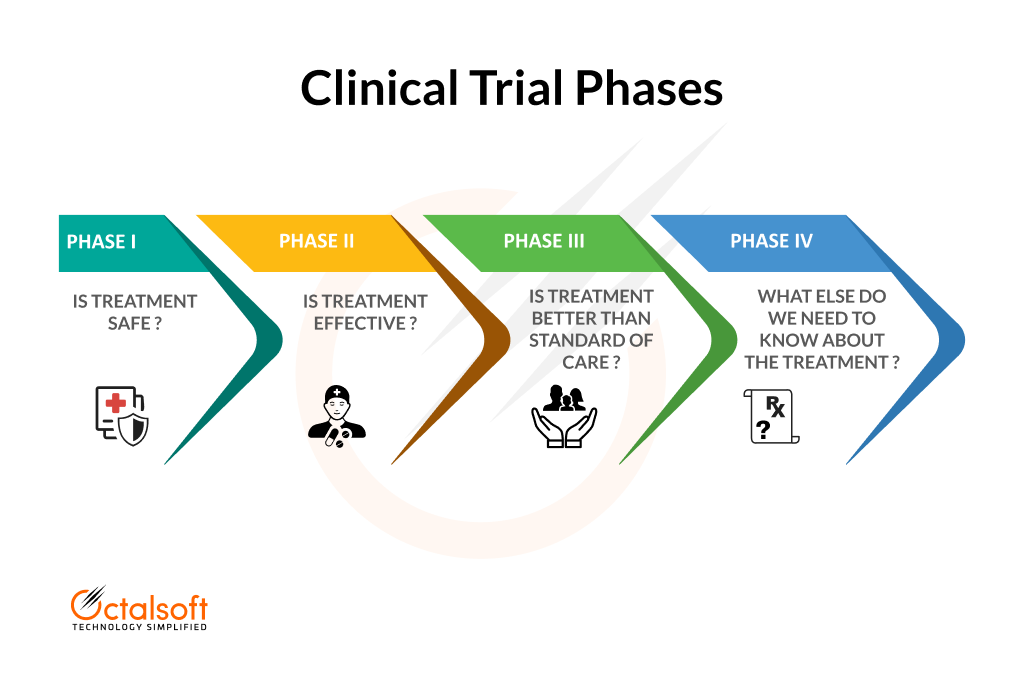
Risk-based monitoring guarantees the quality of clinical trials by identifying, analyzing, monitoring, and reducing any risks to the study's quality or safety. The US Food and Drug Administration (FDA) recommends using a risk-based approach to monitoring in three scenarios:
- To identify important data and procedures.
To correctly manage a research's quality and participant safety, the sponsor must understand which parts are most critical for each trial, ranging from informed consent to eligibility screening and adverse event tracking.
- To perform a risk assessment.
A risk assessment entails identifying particular sources of risk and evaluating the impact of research flaws on those risks.
- To create a monitoring plan.
A monitoring strategy, according to FDA advice, should "describe the monitoring methods, responsibilities, and requirements of the trial." The plan is in charge of explaining hazards and monitoring processes to all parties participating in the experiment.
Why is risk-based monitoring on the rise?
Clinical studies have become increasingly costly and difficult in recent years. The conventional on-site evaluation of trial data can now account for up to one-third of a study's total cost.
This strategy, which is primarily reliant on extensive source data verification (SDV), has shown to be both resource-intensive and restricted in its capacity to detect and avoid problems.
Because efficient monitoring is crucial to preserving trial participants' well-being and ensuring the accuracy of final results, it is widely agreed that the clinical trial monitoring process must evolve.
Several regulatory organizations, including the FDA, now recommend a more centralized, risk-based approach to clinical trial monitoring.
Why is it better?
One key characteristic of risk-based monitoring is the utilization of centralized monitoring methods. Compared to on-site monitoring based on 100% source data verification, centralized monitoring offers a number of advantages:
- Fewer mistakes. On-site monitoring approaches, like any manual effort, are restricted in scope and subject to error. Risk-based, centralized monitoring relies on more automatic evaluations to identify the need for manual intervention, which is more likely to reveal problems.
- Lower the cost. With centralized monitoring, operations such as on-site audits may be confined to study locations where issues are most likely to arise, significantly lowering monitoring costs.
- Better analysis. With all data streaming into a single risk dashboard, statistical and graphical checks may be utilized to quickly identify outliers or unexpected trends in the data.
- Cross-site comparisons. Centralized monitoring also enables you to compare data from several sites to evaluate performance, identify possibly false data, and discover malfunctioning or miscalibrated equipment.
- More timely results. A dashboard also allows you to detect and handle issues as they arise throughout the trial.
“The traditional monitoring process relies on 100% source data verification, which is time-consuming and costly. Here is where RBM makes a difference.”
Krunal Bhatt, Technical Manager, Octalsoft
The Monitoring Process
As previously noted, the FDA has offered thorough instructions on how to develop a monitoring strategy; but, once the plan is in place, it is the duty of the sponsor (or the sponsor's delegate, such as a CRO) to carry it out.
While specifics will vary greatly between research, a risk-oriented monitoring approach would often include the following actions, all of which run through a comprehensive risk dashboard created specifically for your study.
- Data gathering and submission. A centralized strategy necessitates a consistent and dependable flow of information from each research location to the central monitoring system. This can be accomplished either by manually entering and transferring essential data or by establishing an automatic link between the data input system and the central dashboard.
- Dashboard monitoring. The dashboard's purpose is to offer quick access to information regarding the status of each research site in relation to the trial's unique risk variables. When a site has a high risk level, your monitoring strategy should assist you in determining whether more inquiry is necessary, ranging from in-depth statistical analysis to on-site data verification.
- Statistical analysis. In addition to monitoring your risk dashboard, conducting other statistical studies can assist spot concerns. Simple histograms and box plots may be quite useful in identifying outliers between locations or nations for a variety of risk indicators. More sophisticated approaches, such as clustering, can also aid in identifying problematic sites or even fraud.
- Targeted on-site investigation. Dashboard monitoring and further analysis might occasionally indicate that an in-person inquiry is required at a specific site. Depending on the nature of your research, it may be suitable to pay a visit and do a more typical source data verification activity. The crucial point is that, once a centralized system is in place, on-site investigations should be the exception rather than the usual.
The transition to more centralized monitoring offers significant hurdles, but regardless matter where you begin or how gingerly your company adopts the notion, the move is underway. And it's better to be on the cutting edge than to lag behind, especially in an industry where speed to market is critical.
In conclusion
In conclusion, risk-based monitoring represents a modern approach to clinical trial monitoring that takes into account the limitations of traditional monitoring methods.
Sponsors can ensure better data quality, cost-effectiveness, and regulatory compliance by implementing RBM tools, especially in complex, large, adaptive, or resource-intensive trials. Octalsoft's eClinical suite stands out as a powerful solution, offering comprehensive support for RBM and paving the way for more efficient and successful clinical trials. Want to know more about how Octalsoft’s eClinical suite can help you deploy RBM to your next clinical trial? with us today!