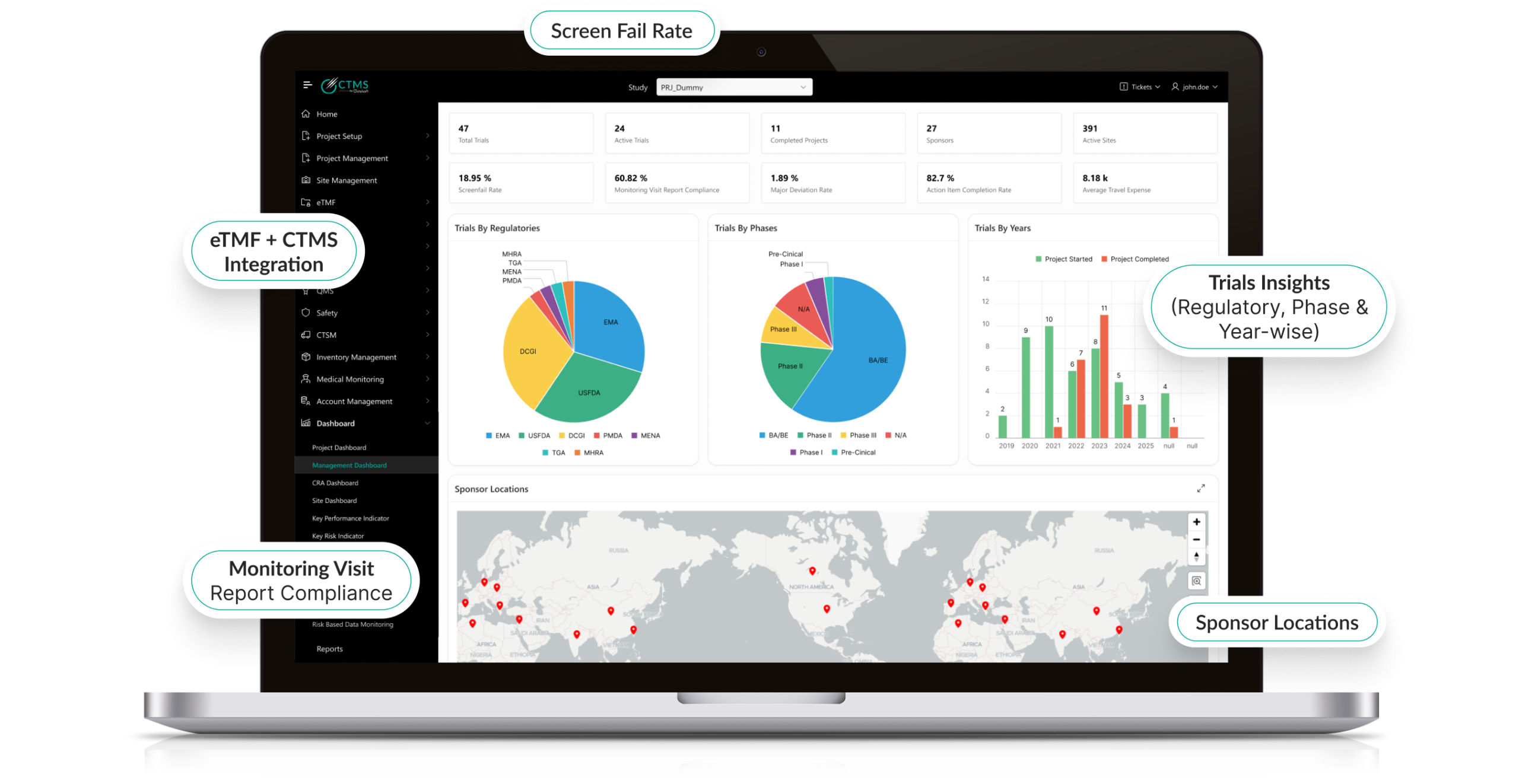
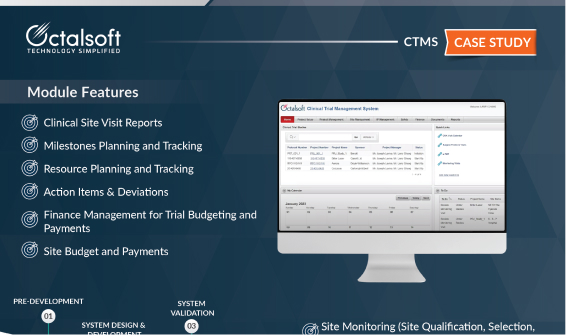
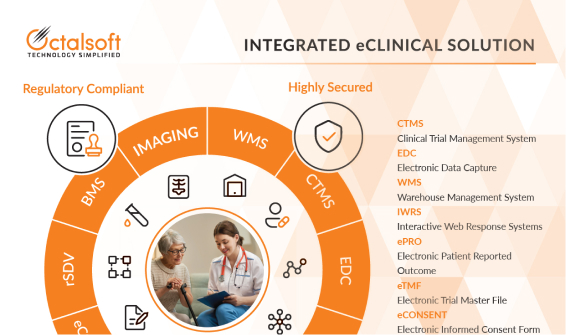
Features & Benefits

Benefits
- Improved Efficiency: Streamline trial processes and reduce administrative burden.
- Enhanced Compliance: Minimize regulatory risks with robust compliance features.
- Faster Decision-Making: Make informed decisions with real-time access to trial data.
- Cost Control: Better manage trial budgets and reduce financial overruns.
- Collaboration and Transparency: Improve communication and transparency with stakeholders.
Features
- Centralized Trial Management: Manage multiple trials from a single, centralized platform.
- Regulatory Compliance: Ensure adherence to regulatory standards with built-in compliance checks and audit trails.
- Real-time Data Access: Access real-time trial data for faster decision-making.
- Advanced Reporting: Generate detailed reports and analytics to monitor trial progress and outcomes.
- Budget and Finance Tracking: Track trial budgets, expenses, and financial milestones.


Benefits
- Increased Productivity: Streamline project management and increase operational efficiency.
- Client Satisfaction: Enhance client satisfaction with real-time updates and transparency.
- Resource Optimization: Optimize resource allocation and reduce operational costs.
- Improved Compliance: Ensure regulatory compliance and reduce audit risks.
- Data-Driven Decisions: Make better decisions with integrated and comprehensive data.
Features
- Project Management: Comprehensive project management tools to oversee multiple trials.
- Client Portal: Secure client portals for real-time updates and collaboration.
- Resource Allocation: Efficiently allocate resources and manage workloads.
- Document Management: Centralized document repository with version control.
- Regulatory Compliance: Built-in compliance checks and audit trails to meet regulatory requirements.


Benefits
- Operational Efficiency: Improve site operations and reduce administrative workload.
- Patient Engagement: Enhance patient recruitment and retention with effective tools.
- Accurate Data Collection: Ensure accurate and timely data capture for better trial outcomes.
- Inventory Control: Efficiently manage clinical supplies and reduce wastage.
- Improved Coordination: Foster better communication and coordination with sponsors and CROs.
Features
- Site Management: Efficient management of site activities and staff.
- Patient Recruitment: Tools to facilitate patient recruitment and retention.
- Visit Scheduling: Streamlined scheduling of patient visits and follow-ups.
- Data Capture: Electronic data capture for accurate and efficient data collection.
- Inventory Management: Track and manage clinical supplies and inventory.

Benefits
- Enhanced Research Efficiency: Streamline study management and improve research efficiency.
- Collaboration and Networking: Foster collaboration and networking among researchers.
- Compliance Assurance: Ensure compliance with regulatory and academic standards.
- Data Protection: Safeguard sensitive research data with robust security features.
- Funding Management: Efficiently manage research grants and funding.
Features
- Study Management: Comprehensive tools for managing academic and clinical studies.
- Collaboration Platform: Facilitate collaboration between researchers and institutions.
- Regulatory Compliance: Ensure compliance with academic and regulatory standards.
- Data Security: Robust data security features to protect sensitive information.
- Grant Management: Manage research grants and funding efficiently.

Frequently Asked Questions
Octalsoft's CTMS streamlines clinical trial operations by centralizing data management, enabling real-time monitoring, improving communication among stakeholders, ensuring regulatory compliance, optimizing resource allocation, and automating workflows. This enhances efficiency, reduces errors, and accelerates study timelines.
Octalsoft's CTMS streamlines clinical trial operations by centralizing data management, enabling real-time monitoring, improving communication among stakeholders.
Yes , Octalsoft provide Support and Training. Octalsoft's CTMS streamlines clinical trial operations by centralizing data management, enabling real-time monitoring, improving communication among stakeholders.
Implementation and Cost Octalsoft's CTMS streamlines clinical trial operations by centralizing data management, enabling real-time monitoring, improving communication among stakeholders.
Have a question we haven’t covered here?
Click below to speak with our experts, ready to answer your queries.
Our Vetted Experience
Related Solutions

EDC
Maintain a centralized, relevant, and most up to date study and operational database; thus providing users with total control, while complying with all regulations.


IWRS
Effectively configure subject enrolment and randomization process and also manage global IP supply chains, using an intuitive web-browser interface.

eTMF
Deploy a highly effective eTMF solution to electronically capture, organize, share, and store all those essential trial documents, images, and artifacts.