Ensuring Global Compliance With:
Core Capabilities of Octalsoft CTSM
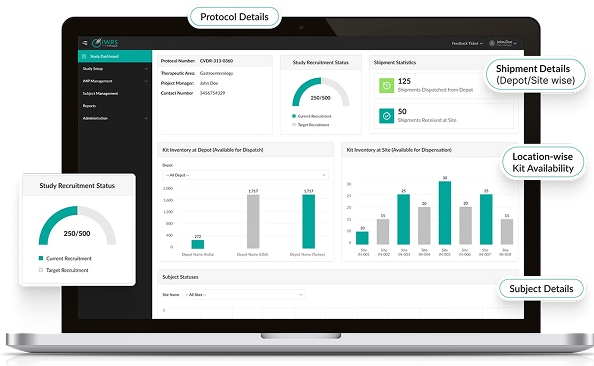
End-to-End Clinical Trial Supply Chain Control
- Centralized oversight of drug packaging, labeling, distribution, and returns.
- AI-enabled dashboards for real-time tracking of clinical trial materials across global depots and sites.
- Ensures traceability, compliance, and chain-of-custody integrity across the clinical supply chain.
Smart Forecasting & Demand Planning
- Predictive AI models account for enrollment trends, randomization schedules, and site performance.
- Minimizes shortages, wastage, and overages across the clinical trial supply chain process.
- Supports adaptive clinical supply forecasting as trials evolve.
Automated Resupply Management
- Dynamic resupply triggered by site consumption and trial milestones.
- Optimizes inventory and shipping frequency, improving clinical trial inventory management.
- Reduces manual interventions and delays in trial supply management.
Temperature & Condition Monitoring
- IoT-enabled sensors safeguard temperature-sensitive supplies.
- AI-driven alerts prevent supply excursions and enable rapid corrective action.
- Protects product integrity and patient safety throughout clinical trial supply logistics.
Returns, Reconciliation & Destruction
- Automated workflows for returned and unused IP.
- End-to-end visibility across clinical supply operations—from distribution to reconciliation.
- Fully compliant with GDP, GMP, GCP, and 21 CFR Part 11 for safe supply destruction.
Compliance-Ready & Inspection-Proof
- Full audit trails and role-based access control.
- Meets international clinical trial supply chain management standards.
- Automated documentation ensures inspection readiness at all times.
Resources That Speak
for Octalsoft CTSM

eClinical Suite Case Study

Factsheet – Clinical Trial Supply Management (CTSM)
Enhance Your CTSM
with These Powerful Add-ons
Octalsoft IWRS / RTSM
Align randomization with drug supply management in clinical trials for seamless end-to-end trial oversight.
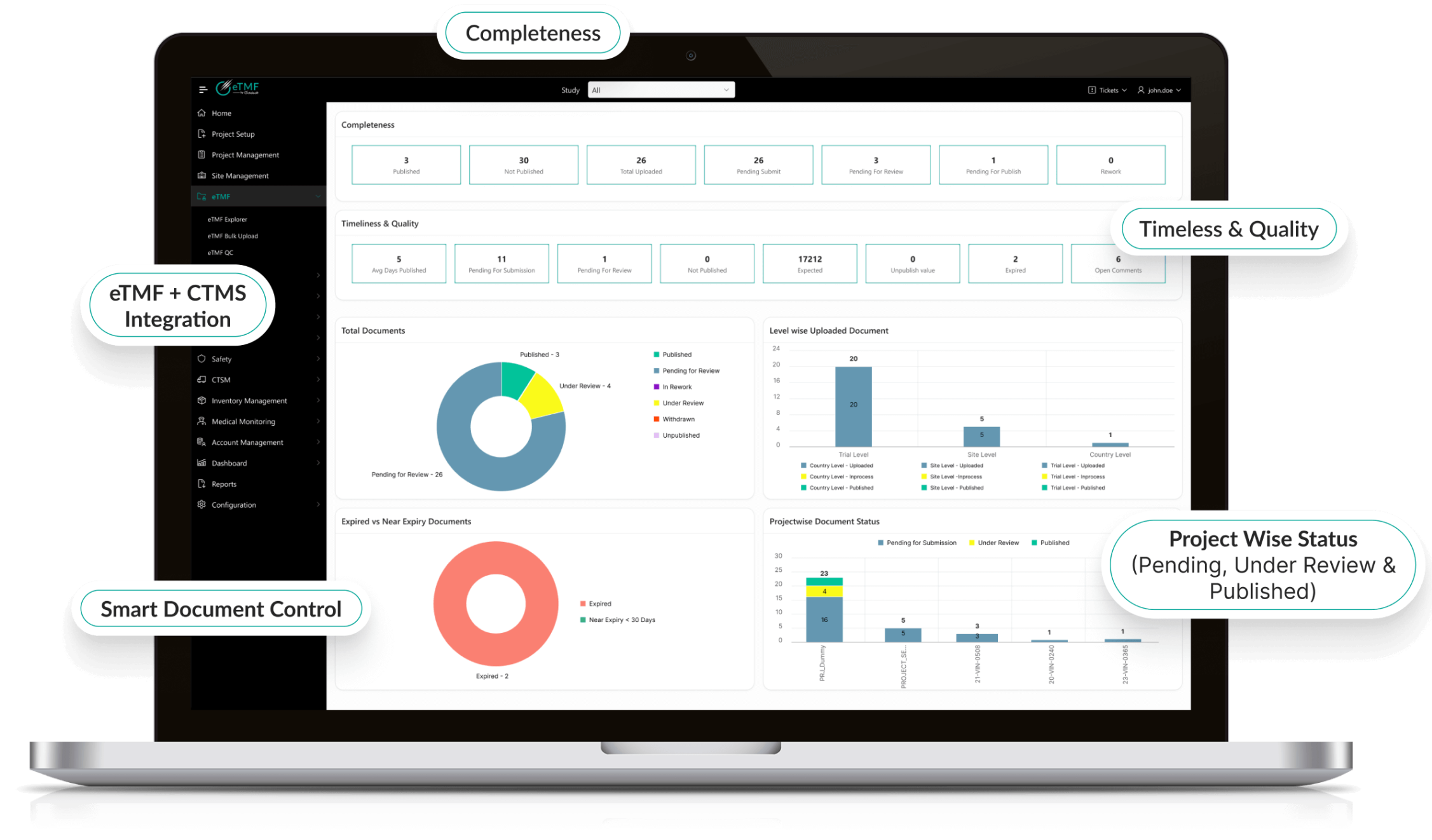
Octalsoft eTMF
Keep all clinical trial supply documentation inspection-ready and fully integrated.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Reduce trial risks and costs with precise forecasting, optimized clinical supply planning, and real-time visibility across global studies.CROs
Manage multi-sponsor, multi-country clinical supplies with streamlined logistics workflows and seamless integration across systems for maximum efficiency.Sites
Ensure timely, reliable deliveries to prevent dosing disruptions and simplify compliance, tracking, and inventory management with advanced clinical supply tools.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download