In the many-sided universe of clinical research, cooperation is the foundation of progress. Lately, a surprising pattern has been picking up speed - cross-sector partnerships. Conventional limits between sectors are obscuring as pharma companies, scholarly establishments, non-profit organizations, and even tech goliaths hold hands to handle the intricacies of clinical research.
Cross-sector partnerships (CSPs) are collaborations between organizations from at least two different societal sectors (i.e. business, government, and nonprofit) that work together in the pursuit of economic, social, and environmental welfare (Bryson et al., 2015; Clarke & Crane, 2018; Selsky & Parker, 2005). The cooperative methodology of collaborative innovation is revolutionizing the scene of clinical research, uniting different abilities to speed up the advancement of life-saving medicines and treatments. How about we dive further into this blossoming pattern and investigate its suggestions for the fate of medical care?
Types of Cross-Sector Partnerships in Clinical Research
Generally, pharma companies have driven the charge in drug advancement, putting vigorously into innovative work (research and development) to put up new treatments for sale to the public. Nonetheless, the drug business faces various difficulties, including rising research and development costs, extended development timetables, and the high disappointment rate of trial drugs.
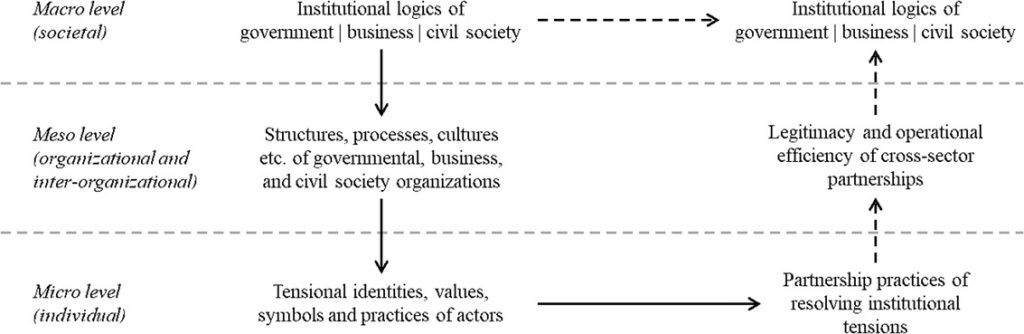
Framework for an institutionalist microfoundation of cross-sector partnerships
Note: Own framework based on Coleman (1990) and Thornton et al. (2012).
"Today, Organizations are progressively coordinating efforts as an essential objective. By banding together with scholarly establishments, non profits, and other industry players, pharma companies can take advantage of an abundance of logical skills, access new innovations, and offer the monetary dangers related to drug development."
Krunal Bhatt, Technical manager Octalsoft
1. Scholarly establishments
Scholarly establishments assume a vital part in propelling clinical exploration, leading essential research that structures the premise of medication development. By teaming up with pharma companies, scholastics get to assets, financing, and certifiable information important to make an interpretation of their revelations into clinical applications. These organizations work with the interpretation of logical forward leaps from the seat to the bedside, facilitating the advancement of imaginative treatments for patients out of luck.
2. Non-profit organizations
Non-profit organizations are likewise vital participants in the cross-sector joint effort scene. Organizations like the Bill and Melinda Doors Establishment, the Michael J. Fox Establishment, and the Public Foundations of Wellbeing (NIH) effectively participate in subsidizing exploration and cultivating organizations to address squeezing medical services difficulties.
By utilizing their assets and organizations, non-profits can catalyze coordinated efforts between different partners, driving advancement and speeding up the speed of clinical advancement.
3. Tech organizations
Tech organizations, especially those in the fields of man-made consciousness (artificial intelligence) and information research are progressively wandering into the domain of medical services and life sciences.
These organizations bring cutting-edge innovations and computational abilities that can upset drug revelation, clinical preliminary plans, and patient consideration. By joining forces with pharma companies and research establishments, tech monsters can apply their aptitude to dissect tremendous measures of information, recognize novel medication targets, and advance treatment regimens, eventually working on quiet results.
Advantages of Cross-Sector Partnerships
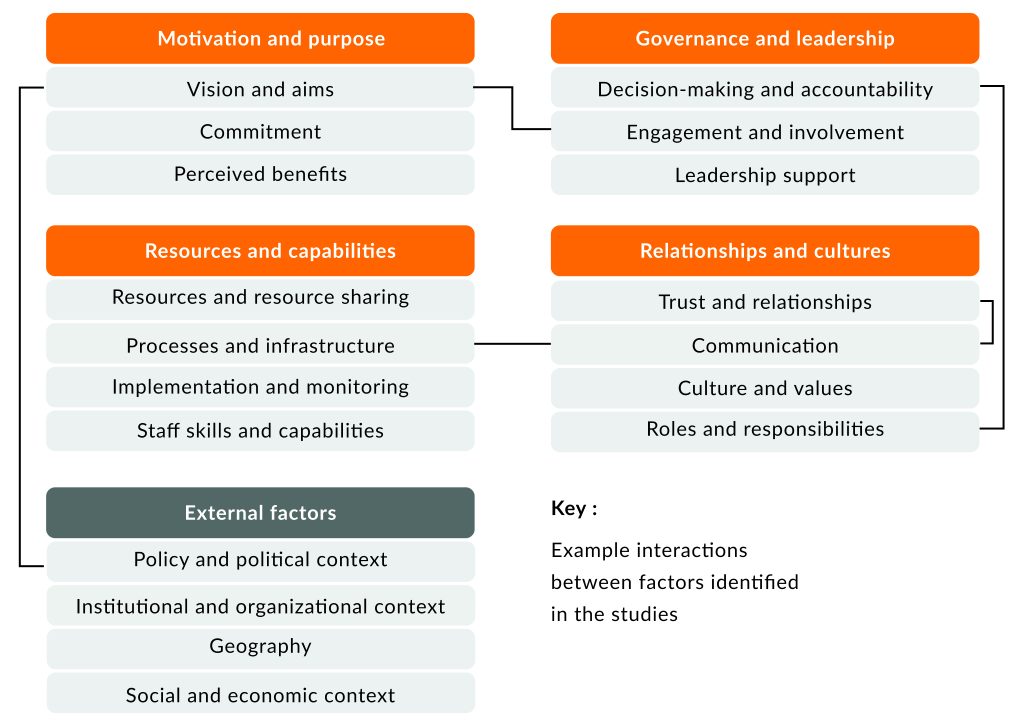
Factors influencing collaboration functioning and example interactions between them
1. A Larger Asset and Skill Pool
One of the main advantages of cross-sector partnerships is the capacity to pool assets and skills, empowering more proficient and savvy drug development.
By sharing information, framework, and dangers, teammates can speed up the speed of advancement and put up new treatments for sale to the public all the more rapidly. Also, organizations can improve the vigor and generalizability of clinical exploration by including different patient populations, geographic locales, and sickness regions.
2. A Culture of Open Collaboration
Cross-sector partnerships advance a culture of open development, where thoughts are uninhibitedly shared, and joint effort is empowered. By separating storehouses and cultivating interdisciplinary cooperation, partners can altogether handle complex logical difficulties that would be hard to address in segregation.
This cooperative methodology drives logical revelation as well as cultivates a feeling of collaboration and mutual perspective inside the exploration local sector.
3. Upgraded Research Quality
Cross-sector partnerships can upgrade the quality and importance of clinical exploration by consolidating different points of view and needs. By including patients, parental figures, support gatherings, and administrative offices in the research cycle, teammates can guarantee that clinical preliminaries are planned in light of the end client, prompting more understanding-focused results and further developed medical care conveyance.
4. Better Disease Research
One region where cross-sector partnerships are especially significant is in uncommon illness research. Interesting sicknesses frequently present one-of-a-kind difficulties because of little quiet populaces, restricted comprehension of illness components, and an absence of accessible therapies.
By teaming up across sectors, specialists can conquer these hindrances by pooling information, assets, and mastery to speed up the development of treatments for intriguing sicknesses.
Patient promotion bunches likewise assume a basic part in these organizations, giving significant bits of knowledge into the lived encounters of patients and guardians, directing research needs, and working with patient enrollment for clinical preliminaries.
As well as propelling medication advancement, cross-sector partnerships are likewise driving development in clinical preliminary plans and execution.
By tackling advancements like wearable gadgets, telemedicine, and remote checking, specialists can gather continuous information and lead preliminaries all the more productively and comprehensively. These developments smooth out the preliminary cycle as well as work on quiet commitment, maintenance, and by and large review results.
Challenges of Cross-Sector Partnerships
Regardless of the many advantages of cross-sector partnerships, challenges remain. Contrasts in authoritative culture, needs, and motivations can once in a while frustrate powerful joint effort.
Protected innovation concerns, administrative boundaries, and irreconcilable circumstances may likewise emerge, requiring cautious exchange and administration systems to explore. In any case, by cultivating a feeling of trust, straightforwardness, and common perspective, partners can defeat these difficulties and tackle the maximum capacity of cooperative development in clinical exploration.
The Path Ahead
Looking forward, the eventual fate of clinical exploration is without a doubt interlaced with cross-sector partnerships.
As the speed of logical revelation speeds up and the medical services scene keeps on advancing, the joint effort will be more basic than at any other time in driving advancement and working on persistent results.
By embracing a cooperative outlook and manufacturing organizations across sectors, we can open new doors for development and usher in another time of customized medication, where each quiet approaches the medicines they need to reside better, longer resides.
In Summation
All in all, cross-sector partnerships are a strong power for driving development in clinical exploration. By uniting different partners, including pharma companies, scholastic foundations, non-profit organizations, and tech organizations, these organizations can speed up drug advancement, upgrade the quality and significance of clinical exploration, and work on persistent results.
As we keep on exploring the intricacies of clinical exploration and medical services conveyance, the joint effort will be critical to opening new revelations and changing the fate of medication.
At Octalsoft, we have already begun stepping into the future with our comprehensive suite of eClinical software solutions. Do you want to know how you can overcome existing clinical trial challenges, accelerate the development of innovative therapies, and usher in a new era of evidence-based medicine that benefits patients worldwide with Octalsoft?



