In the rapidly changing landscape of clinical research, effective data capture and management make the difference in a successful clinical trial. Maintaining integrity, accuracy and regulatory compliance is more than a technical issue. It’s a strategic imperative. Two systems are central to this process, electronic data capture (EDC) and clinical data management system (CDMS). Although these terms are on occasion used synonymously, they’re not the same and they play separate roles in the clinical trial lifecycle.
In this blog, we’re diving into the distinctions between EDC vs. CDMS, why it matters, and how they complement each other in ensuring quality and compliance in today’s clinical trials.
What’s EDC?
Electronic Data Capture (EDC) is software which allows clinical trial sites to record data electronically, as opposed to on paper CRFs. These systems are generally online and enable investigators, study coordinators and monitors to enter and review data in real time.
EDC systems are mainly meant for data capture. They provide built eCRFs where such data can be input and securely stored. Embedded logic provides that invalid or inconsistent data entries are flagged up on entry with automatic edit checks. Users are able to monitor changes and answer data requests inside the system, preserving an audit trail that enables regulatory compliance.
The key benefits of EDC systems are quicker data entry, fewer errors, access to data in real-time for remote teams and more efficient query handling. These systems provide an order-of-magnitude gain in data quality and monitoring efficiency over paper.
What is CDMS?
A CDMS is a more expansive solution that oversees the entire lifecycle of clinical trial data. There’s data capture, as a piece–typically via a native or connected EDC–but it goes way beyond that to encompass data cleaning, reconciliation, coding, tracking, validation and export for stats analysis.
CDMS platforms are generally employed by data managers, biostatisticians and regulatory teams to prepare the data collected — ensuring it is accurate, clean and ready for submission to health authorities. A CDMS manages things like medical coding with standard dictionaries (MedDRA, WHODrug), discrepancy and query management across multiple sources of data, and the reconciliation of data from labs, ePRO systems, imaging platforms, and even wearables.Besides the data quality management, a CDMS offers reporting dashboards, status tracking, data review workflows, and the tools necessary to lock and freeze datasets at important milestones. The platform supports compliance with international standards like ICH GCP and 21 CFR Part 11, which are crucial for regulatory filings.
How EDC and CDMS Work Together
Although EDC and CDMS are distinct systems, they are often deployed in tandem. In fact, many modern clinical trial platforms offer a unified environment where EDC is one module within a broader CDMS framework. Here’s how they typically interact during a trial:
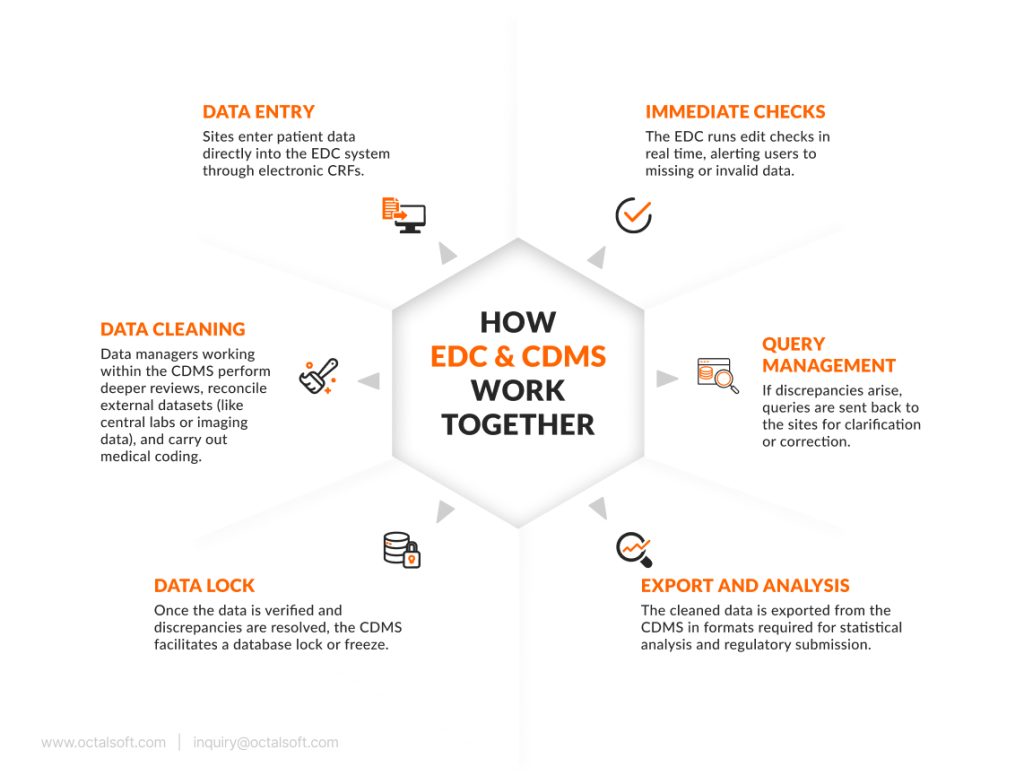
This workflow illustrates that while EDC systems are essential for collecting structured, high-quality data at the site level, CDMS platforms are responsible for turning that raw data into a validated, submission-ready dataset.
Knowing the Fundamental Distinctions
At a high level, the difference between EDC and CDMS is scope and purpose.
EDC was exclusively centered on collecting clinical trial data electronically at the point of care. It substitutes paper forms with a digital interface and ensures site staff inputs patient data accurately and efficiently. EDC aims to simplify the data entry process, minimize errors, and provide real-time data faster.
CDMS is a broader platform that includes EDC but offers a complete set of tools to handle and process data. It backs the whole data wrangling pipeline from collection and cleaning to validation and export. If EDC is about collecting the data, CDMS is about wrangling it until it’s analysis-ready.
One alternative way to parse the distinction is to examine who uses each system. EDC is the usual way that site staff, investigators, and monitors enter and browse patient data. CDMS is primarily employed by data managers, biostatisticians and regulatory teams, who are tasked with ensuring that the data is complete and correct, and preparing it for statistical analysis and submission.
The Convergence of EDC and CDMS
It’s interesting to observe that in recent years, the distinction between EDC and CDMS has started to become fuzzy. These modern clinical trial platforms, including Medidata Rave, Oracle Clinical One, and Veeva Vault CDMS, provide modular, integrated systems that couple EDC with a comprehensive suite of data management tools. These solutions offer centralized user interfaces, common dashboards, and smooth integration across data sources.
This convergence is fueled by the intricate nature of modern-day trials that include decentralized sites, remote data capture, wearables, and real-time patient engagement tools. In these spaces, a piecemeal data strategy is no longer enough. Unified platforms that nest EDC within a larger CDMS framework provide the scalability and agility that today’s research requires.
Summary
In a nutshell, as critical to the clinical trial paradigm as both EDC and CDMS are, they cover different, complementary grounds. EDC is about capturing patient data more efficiently and accurately, whereas CDMS is about managing, cleaning, validating and preparing that data for analysis and regulatory submission.
How you choose your system—or systems—really depends on how complicated your study is, what kind of data you’re collecting, and your regulatory requirements. Whether you’re conducting a small Phase I trial or a massive, global Phase III program, knowing the EDC vs. CDMS distinction is key to establishing a strong, compliant, and efficient data management plan.
If you’re planning your next trial and looking for expert advice on the right data management tools, our team can help. Contact us today to discuss solutions uniquely suited for your protocol, sites, and study design. Want to expedite your next clinical study with a feature-packed, hyper- accurate EDC?