In today’s fast-moving world of clinical research, where timelines, accuracy and regulatory compliance are paramount, a CTMS has become the foundation of trial operations. Whether conducting a single-site study or managing multi-deep global trials, CTMS software is the digital scaffolding that allows frictionless collaboration, centralized control, and regulatory preparedness.
So what is CTMS, and why is it essential in today’s clinical trial ecosystem? Read on.
What exactly is CTMS?
CTMS means Clinical Trial Management System, which is the software platform used by sponsors, CROs, and clinical sites for planning, executing, and tracking clinical trials.
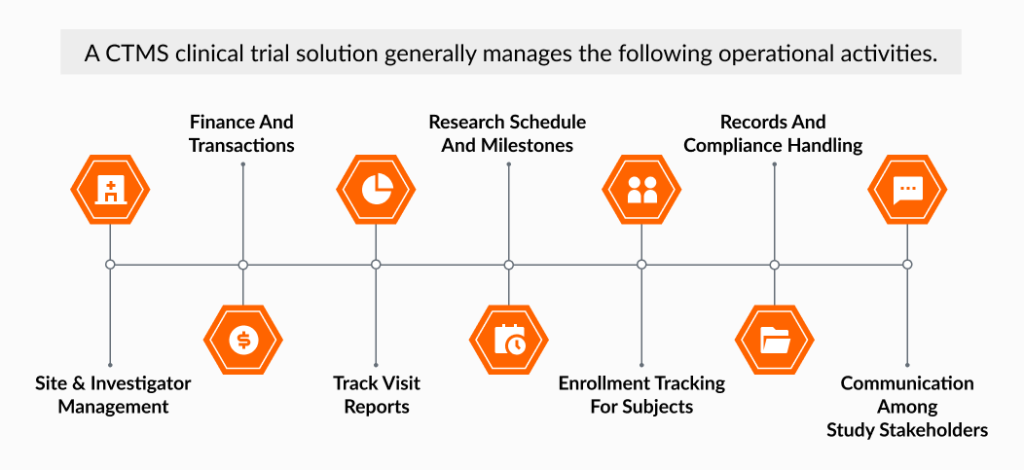
Basically, CTMS systems are the digital nerve center of a clinical trial — bringing all operational data and documents into one place to streamline operations and facilitate compliance with global regulations like ICH-GCP and FDA 21 CFR Part 11.
Why CTMS is Mission Critical
Here’s why you need a CTMS for clinical trials that are data-rich, highly regulated, and increasingly global.
1. Centralized Oversight
With multiple sites, investigators, and timelines to juggle, centralization is crucial. A good CTMS provides real-time visibility into study progress across geographies and teams, enabling data-driven decisions and early risk mitigation.
2. Real-time CTMS for nimble operations
Modern trials require agility. Realtime CTMS solutions allow sponsors and CROs to monitor site performance and subject enrollment and receive notifications of deviations or delays. This real-time feedback loop enables teams to course-correct before issues fester.
3. Improved regulatory compliance
Audits and inspections will occur. CTMS platforms offer audit trails, document versioning, and compliance checklists that keep teams inspection-ready around the clock.
4. Easy Budgeting and Payments
Tracking investigator fees, milestone-based payments, and financial reconciliations across 100’s of sites by hand is a disaster waiting to happen. CTMS systems automate this process with transparency and control.
Key Features of Modern CTMS Systems: What should modern CTMS systems have?
Numerous platforms purport to provide CTMS functionality; not all are made equal. Here’s what to watch for in a modern CTMS clinical trial solution.
- Customizable Dashboards—real-time visualizations for trial KPIs and milestones.
- Site & Investigator Management: Central repository for credentials, performance, and communications
- Plan, log, and report visits
- Built-in Document Management: Save protocols, contracts, IRB approvals, and training logs
- Track enrollment rates vs. targets for all sites
- Field teams require access on the move, and mobile-optimized CTMS platforms enhance efficiency.
- Role-Based Access Control Make sure users only see what they have to, depending on their role.
A few enterprise solutions, such as Oracle CTMS, have long been industry standards but are expensive and have a high learning curve. That’s why the market has recently witnessed an influx of agile, user-friendly, cloud-native CTMS platforms for today’s clinical trials.
AI-powered, cloud-native CTMS
The CTMS landscape is changing quickly. The next generation of platforms is focusing on
- AI: For predictive analytics and automated alerts
- For scalability, flexibility, and faster deployments
- Native, seamless integrations with EDC, eTMF, and IWRS
- Self-serve configuration, allowing non-technical users to configure studies, workflows, and reports
With these innovations, CTMS solutions aren’t merely utilities. They’re strategic enablers of clinical innovation.
Why Octalsoft CTMS
We know the challenges of modern clinical trials at Octalsoft. That’s why we build our CTMS software to be both robust and flexible. Whether you're a global sponsor or an emerging biotech, our CTMS platform gives you the power to.
- Real-time dashboards and analytics.
- Effortless integrations with EDC, IWRS, and eTMF
- Easy to use with minimal training
- Role-based access for secure and compliant operations
- Automated payment workflows and tracking
Our CTMS systems are relied on by research organizations globally to provide speed, precision, and command at every phase of the clinical journey.
Ready to Experience the Power of Real-Time CTMS?
Related Blogs:
Who Uses Clinical Trial Management Platforms (CTMS)?
4 Questions to Ask CTMS Vendors to Ensure You Get a Good Product




