In today's world, data security is more important than ever. With the ever-increasing number of cyberattacks, organizations must take steps to protect their data. One of the most important ways to do this is to implement a robust security solution.
An electronic trial master file (eTMF) is a critical component of clinical trials. It contains all of the documents and data related to a clinical trial, from the protocol to the final report. As such, it is a valuable target for cyberattacks.
A secure eTMF platform can help to protect your data from unauthorized access, modification, or destruction. It can also help to ensure that your data is compliant with regulatory requirements.
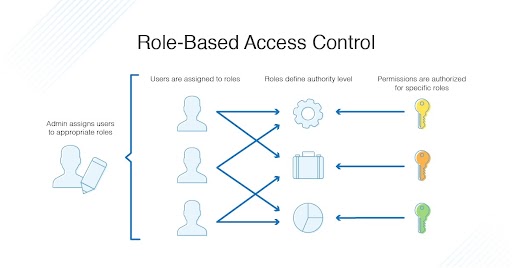
One of the most important features of a secure eTMF platform is role-based access control (RBAC). RBAC allows you to define different roles for different users, and to grant each role specific permissions to access data. This helps to ensure that only authorized users have access to sensitive data.
For example, you could create a role for clinical trial managers that allows them to view and edit all of the data in the eTMF. You could also create a role for data analysts that allows them to view and analyze the data, but not to edit it.
RBAC can also be used to control access to specific documents or data sets. For example, you could create a role for regulatory auditors that allows them to view all of the data in the eTMF, but only for specific trials.
The Benefits of Role-Based Access Control
Role-based access control (RBAC) is a security mechanism that allows you to define different roles for different users, and to grant each role specific permissions to access data. This helps to ensure that only authorized users have access to sensitive data.
There are many benefits to using RBAC in an eTMF platform. Some of the most important benefits include:
Protecting Sensitive Data: One of the primary reasons for implementing security measures in an eTMF platform is to protect sensitive data.
Clinical trial documentation often contains confidential and proprietary information related to trial participants, protocols, and drug development.
Breaches in security can lead to unauthorized access, data manipulation, or even theft of valuable intellectual property. By ensuring robust security measures, such as encryption, firewalls, and intrusion detection systems, eTMF platforms safeguard sensitive data from external threats.
Compliance with Regulatory Standards: The field of clinical research is highly regulated, with numerous guidelines and standards to adhere to. A well-designed eTMF platform with robust security measures can facilitate compliance with regulatory requirements, such as Good Clinical Practice (GCP) guidelines, the Health Insurance Portability and Accountability Act (HIPAA), and the General Data Protection Regulation (GDPR).
These regulations mandate the protection of participant privacy and the secure handling of clinical trial data. By implementing security controls and role-based access, eTMF platforms provide an auditable trail, ensuring regulatory compliance throughout the trial lifecycle.
Mitigating Internal Risks: While external threats pose a significant risk, internal risks should not be overlooked. Not all individuals involved in a clinical trial require access to all documentation. Role-based access control (RBAC) allows eTMF platforms to assign specific roles and permissions to users based on their responsibilities.
This ensures that only authorized personnel can access relevant information, reducing the risk of accidental data breaches or unauthorized data manipulation. RBAC also enables better traceability and accountability, as access and actions within the eTMF platform can be attributed to specific individuals.
Streamlining Collaboration: Clinical trials involve a multitude of stakeholders, including investigators, sponsors, monitors, and regulatory authorities.
Effective collaboration among these stakeholders is crucial for the smooth conduct of trials. Security and RBAC enable controlled sharing and collaboration within an eTMF platform, allowing authorized users to access and collaborate on relevant documents while maintaining confidentiality.
By implementing granular access controls, eTMF platforms provide a secure environment where stakeholders can collaborate efficiently, reducing the administrative burden associated with manual document sharing and version control.
Enhanced Data Integrity: Data integrity is paramount in clinical trials. Any compromise in the integrity of trial documentation can have severe consequences, including compromised participant safety and unreliable study outcomes.
Robust security measures in eTMF platforms, such as data encryption, digital signatures, and audit trails, help ensure the integrity of trial documentation. RBAC further strengthens data integrity by preventing unauthorized alterations or deletions.
With these safeguards in place, eTMF platforms maintain the accuracy, completeness, and consistency of trial documentation, facilitating reliable data analysis and regulatory submissions.
Improved Efficiency and Productivity: Implementing security measures and RBAC in an eTMF platform can significantly enhance efficiency and productivity in clinical trials.
Authorized users can quickly access the information they need, reducing the time spent searching for documents or waiting for manual approvals.
RBAC allows for streamlined workflows, ensuring that tasks and responsibilities are properly assigned, tracked, and executed.
With increased efficiency, stakeholders can focus more on core trial activities, leading to faster study timelines, reduced costs
To implement RBAC in an eTMF platform, you will need to:
- Define the roles that you need in your organization. For example, you might need roles for clinical trial managers, data analysts, and regulatory auditors.
- Assign permissions to each role. For example, you might grant clinical trial managers permission to view and edit all of the data in the eTMF, while you might only grant data analysts permission to view and analyze the data.
- Configure your eTMF platform to use RBAC. This will involve creating user accounts for each role and then assigning permissions to each user account.
Once you have implemented RBAC in your eTMF platform, you can start to enjoy the benefits of increased security, improved efficiency, and reduced risk.
Conclusion
Security and role-based access are essential for eTMF platforms. By implementing a secure eTMF platform, you can help to protect your data from unauthorized access, modification, or destruction. You can also help to ensure that your data is compliant with regulatory requirements.
But not every eTMF platform can handle the complex requirements of a modern eClinical trial. Rest assured, we have your back. Introducing Octalsoft’s eTMF, a feature-rich system that guarantees data security along with easy access and analysis. Want to know more about Octalsoft’s eTMF? Book a Demo with us Now!



