Greetings, fellow supporters of advancement in science and technology! With this blog, we're putting on our diversity goggles and studying how inclusiveness plays a critical part in determining the future of medical developments as we delve into the intriguing realm of clinical research in modern times.. Now put on your lab coat and let's go on this fascinating adventure!
Imagine that a novel medical discovery is about to transform the range of available treatments for a common illness. But wait a minute... who are the research participants? Do they adequately reflect the heterogeneous human fabric, or are we losing out on important information by continuing to use the same old homogenous sample pool? Here are is a statistic that could help offer a clearer picture of the current situation-
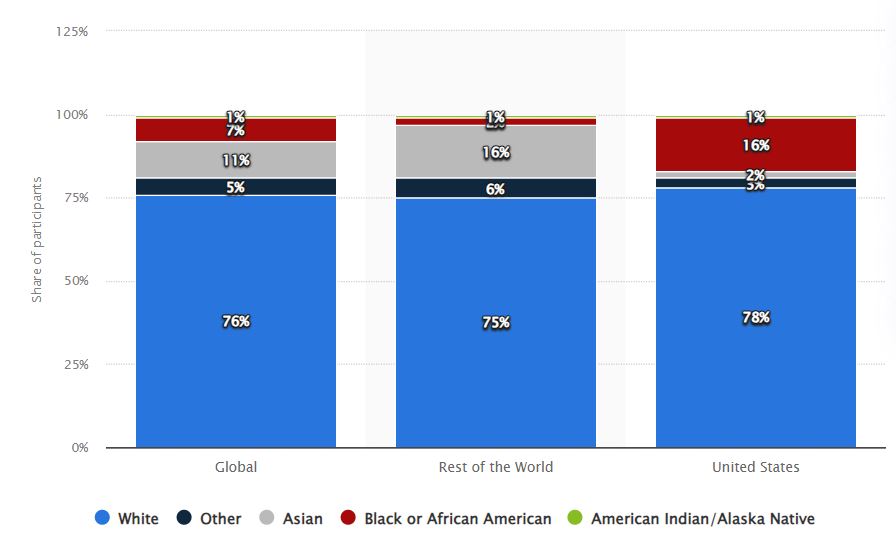
Diversity in clinical research isn't just a buzzword; it's a necessity. Why, you ask? Well, let me hit you with some facts that'll make you sit up and take notice:
- Underrepresentation Conundrum: Were you aware that older people, women, and members of ethnic minorities are frequently underrepresented in clinical trials? Yes, it is accurate! Minorities, such as African Americans, Hispanics, and Asians, only make up a small percentage of clinical study participants, according to an FDA analysis. Not to mention the dearth of gender diversity in trials; women are frequently either overwhelmingly underrepresented or omitted completely.
- Health Disparities Amplified: Were you aware that older people, women, and members of ethnic minorities are frequently underrepresented in clinical trials? Yes, it is accurate! Minorities, such as African Americans, Hispanics, and Asians, only make up a small percentage of clinical study participants, according to an FDA analysis. Not to mention the dearth of gender diversity in trials; women are frequently either overwhelmingly underrepresented or completely omitted.
"Imagine the groundbreaking discoveries we could unlock if we had a more diverse pool of participants in clinical trials. By including individuals from various racial, ethnic, and socioeconomic backgrounds, we gain a more comprehensive understanding of how different populations respond to treatments. It's like assembling a superhero squad with a diverse set of powers - together, they can conquer any challenge!"
Krunal Bhatt, Technical Manager, Octalsoft
So how can we address this diversity issue and create the conditions for a more diverse clinical research community going forward? Do not be alarmed, readers; we have answers for you:
- Outreach and Community Involvement: Let's take the research onto the streets! Building trust and being actively involved in local communities are necessary to draw in a large participation pool. By means of outreach initiatives and partnerships with community groups, we can ensure that all voices are heard at the research table.
- Culturally Tailored Approaches: No one size fits all, especially when it comes to healthcare. Including cultural nuances and preferences in study design may substantially improve the ability to engage a diverse range of participants. To really engage in anything from language to eating traditions, one must comprehend and value cultural variation.
- Representation Matters: It is essential that clinical research take up the mantra "representation matters." To do this, it will be necessary to aggressively seek out, retain, and value volunteers from underrepresented groups and to ensure that their voices are heard throughout the whole research. After all, everyone ought to be able to contribute to the advancement of knowledge!
- Openness and Availability: Let's remove the veil of secrecy around clinical research! It is imperative that study techniques, risks, and rewards be communicated in a way that is easily understood by all parties in order to get informed consent. In addition, genuine inclusion necessitates that research opportunities be accessible to individuals with disabilities as well as those from all backgrounds.
- Policy and Regulation: Last, but no less significant, lawmakers and regulatory agencies play a critical role in promoting diversity and inclusivity in clinical research. Policies that support diversity in study recruiting and funding activities should be implemented in order to close the gap and advance a more equitable research environment.
In Summation
To sum up, people, diversity, and inclusiveness are the pillars of real advancement in clinical research—not just boxes to check off. We can realize the full potential of scientific discovery and make sure that healthcare advances benefit everyone, not just a chosen few, by embracing the rich tapestry of human variation. Thus, let's get our hands dirty, dismantle obstacles, and move forward toward a day where everyone's health is important and their voice is heard. That's what science is all about, after all.
Want to know more about how Octalsoft’s eClinical suite can augment the patient-centricity of your next clinical trial? Get in touch with us now!



