Ensuring Global Compliance With:
Core Capabilities of Octalsoft PPM
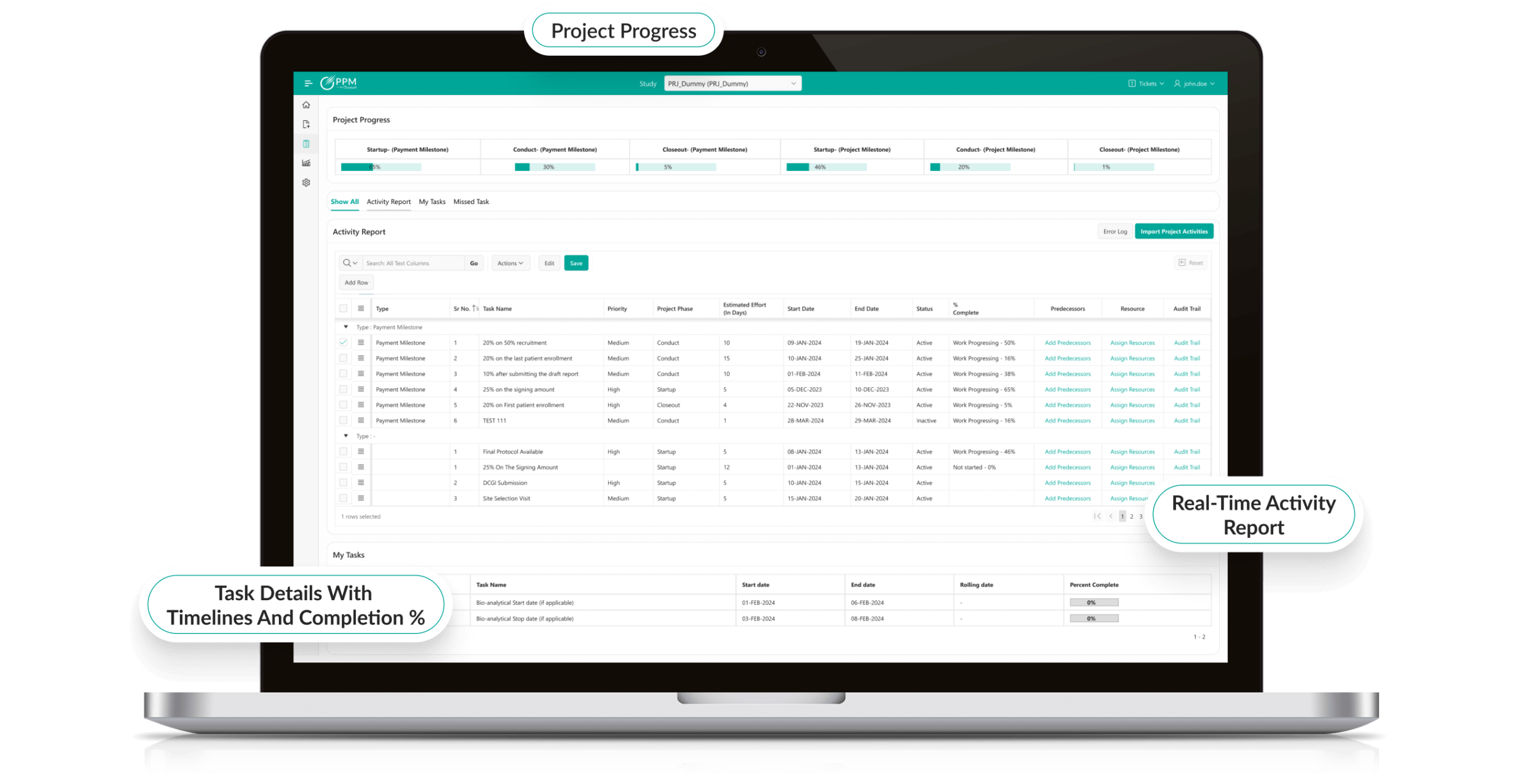
Unified Project Planning
- Create, manage, and monitor projects in a single centralized PPM system.
- Gantt charts, Kanban boards, and milestone tracking for visual clarity.
- Adaptive scheduling that adjusts to trial protocols and enterprise demands—ideal for clinical trial project management software needs.
Portfolio Oversight & Prioritization
- Track multiple programs across geographies and business units.
- Set priorities based on ROI, risk, and strategic alignment.
- Monitor trial pipelines, operational initiatives, and R&D portfolios in one view with robust PPM software solutions.
AI-Driven Resource Management
- Predict and allocate resources across projects to avoid bottlenecks.
- Optimize workforce utilization across business and clinical functions.
- Scenario modeling to plan for growth and contingencies in project management software for pharmaceutical industry.
Budget & Financial Tracking
- Automated budgeting tools with variance tracking.
- Forecast project costs and allocate funds strategically.
- Integrated financial dashboards for executive oversight in a secure cloud-based PPM.
Collaboration & Workflow Automation
- Built-in communication tools for cross-team alignment.
- Custom workflows for protocol amendments, approvals, and change requests.
- Secure document sharing with role-based access.
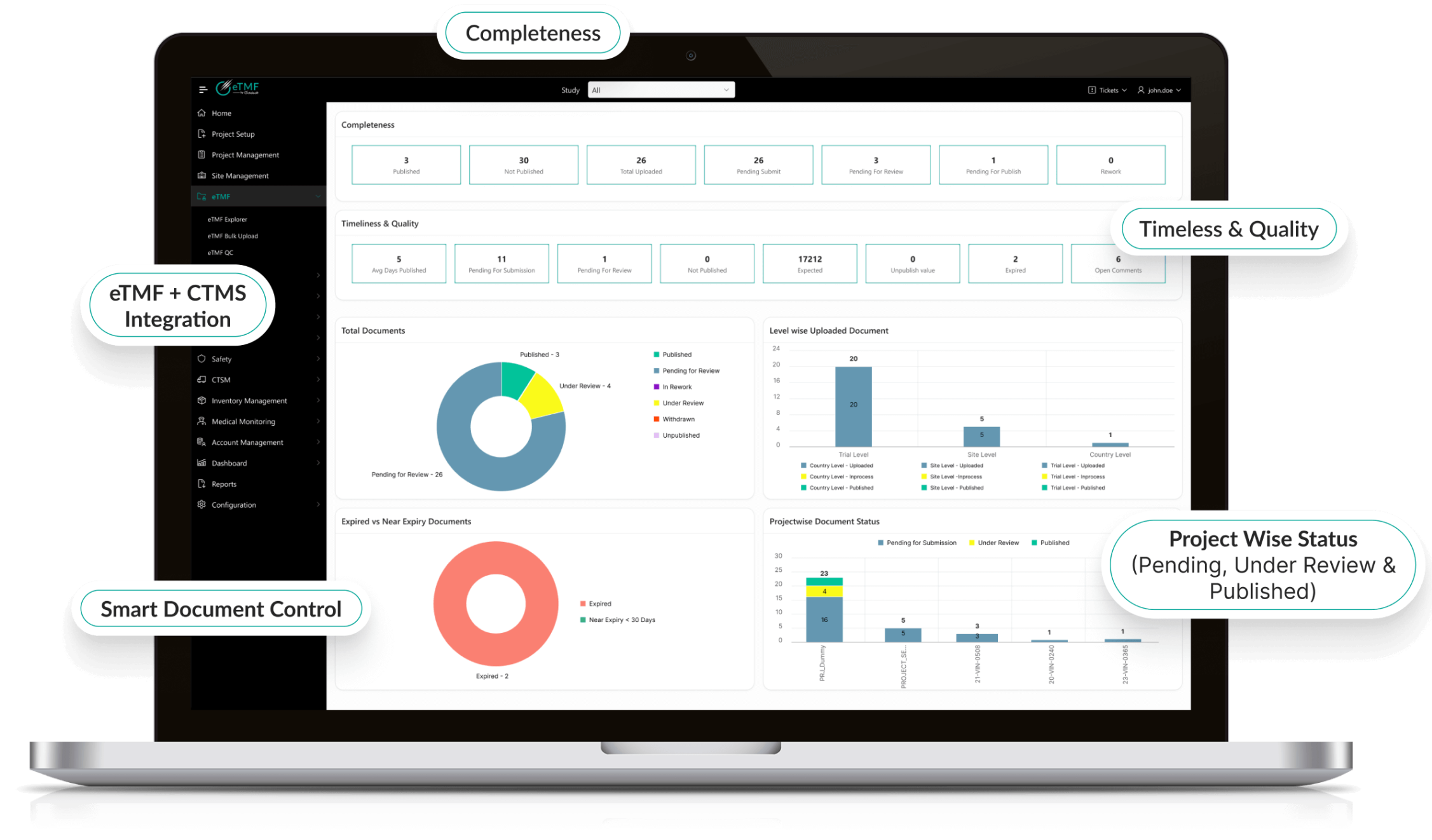
Analytics & Reporting
- Real-time dashboards for KPIs, milestones, and performance metrics.
- Predictive analytics for risk detection and proactive decision-making.
- Regulatory-compliant reporting for sponsors, CROs, and internal stakeholders.
Resources That Speak
for Octalsoft PPM

eClinical Suite Case Study

Factsheet – Portfolio & Project Management (PPM)
Enhance Your CTMS
with These Powerful Add-ons
Octalsoft CTMS
Connect project management with trial operations for complete oversight.
Key Benefits for Every Role
Business Leaders
Align organizational strategy with execution. Drive higher ROI across portfolios using pharma project management software.Clinical Trial Managers
Keep multi-site, multi-phase studies on track with clinical trial project management software for real-time oversight.Project Teams
Collaborate seamlessly, automate tasks, and minimize rework with PPM software solutions.Finance & Compliance Officers
Track costs, ensure regulatory adherence, and generate audit-ready reports with pharmaceutical project management software.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download