Ensuring Global Compliance With:
Our Journey. Our Purpose. Your Progress.
15+
Years Experience
25+
Countries Covered
1.5M+
Lives Touched
40K+
Platform Users
8K+
Clinical Sites
25+
Therapeutic Areas
Since 2007, Octalsoft has been helping life sciences companies, sponsors, sites and CROs accelerate drug development through innovative clinical research software solutions. With a mission to simplify clinical research, we combine deep industry expertise with cutting-edge technology to enable faster decisions and better outcomes. Every milestone we reach is driven by a commitment to empower sponsors, CROs, and sites to achieve progress in clinical trials.

INDUSTRY RECOGNITION IN 2024 AND 2025
Ranked as a Major Contender by Everest Group
For two consecutive years—2024 and 2025—Octalsoft has earned the rank of “major Contender” in Everest Group’s Life Sciences PEAK Matrix® , underscoring its consistent performance and growing market impact as a trusted provider of eClinical solutions, delivering scalable technology designed to support complex global trials.
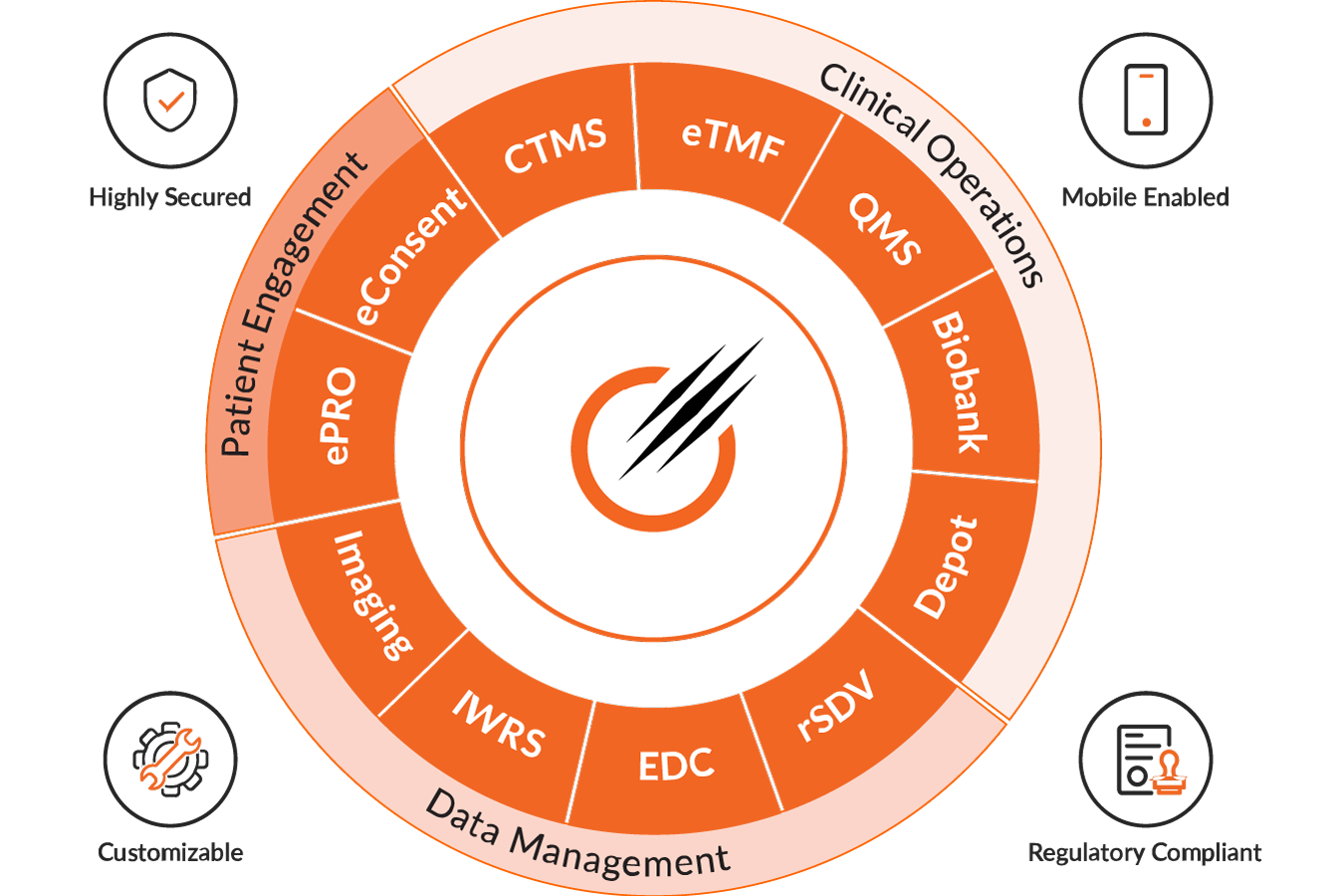
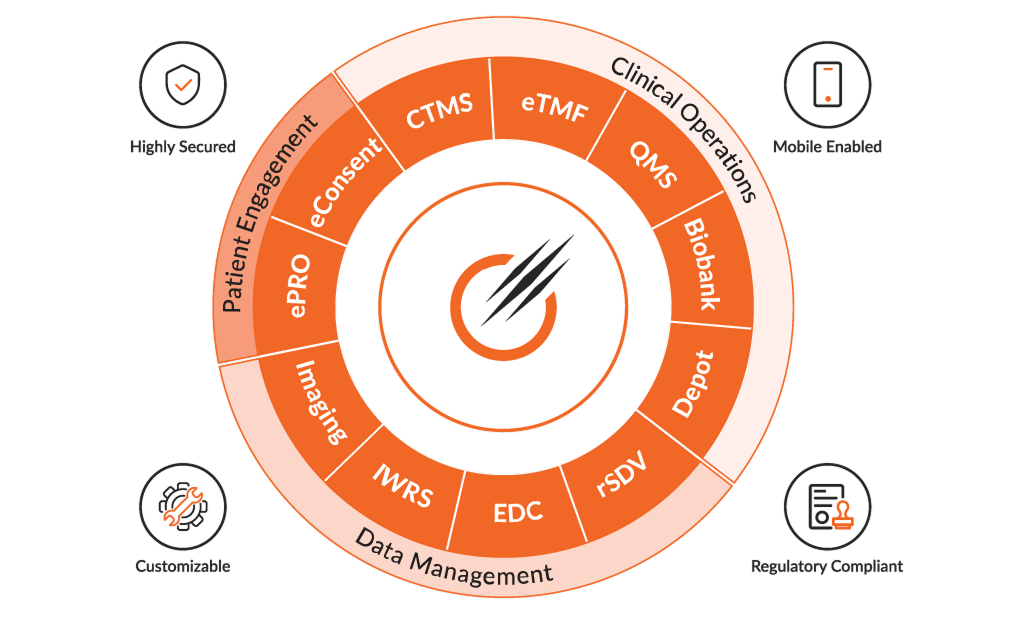
Empowering your success with our solutions
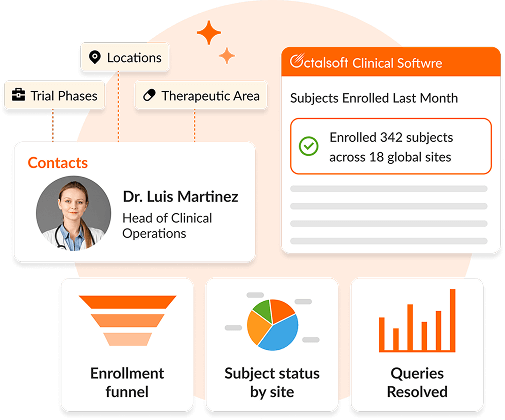
Octalsoft CTMS
A cloud-based Clinical Trial Management System delivering centralized, real-time data tracking, monitoring, and streamlined study execution.
Read MoreOctalsoft EDC
An advanced Electronic Data Capture platform enabling faster study builds, cleaner datasets, and accelerated time to database lock.
Read MoreOctalsoft IWRS
A robust Interactive Web Response System supporting randomization, drug supply forecasting, and secure, compliant patient enrollment management.
Read MoreOctalsoft eTMF
An Electronic Trial Master File solution with metadata tagging, intelligent workflows, and real-time oversight for regulatory-ready document management.loream ipsum is dummy text
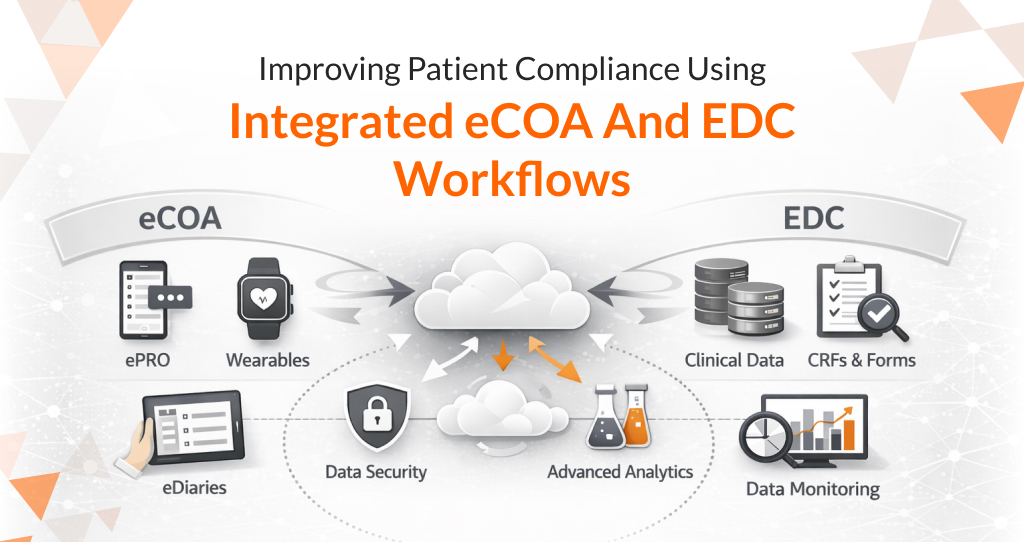
Read MoreOctalsoft ePRO/eCOA
An Electronic Patient Reported Outcome system designed for accurate, real-time patient data capture and enhanced trial compliance worldwide.
Read MoreOctalsoft rSDV
A Remote Source Data Verification tool that strengthens oversight, reduces monitoring costs, and ensures higher data quality in trials.
Read MoreOctalsoft QMS
A Quality Management System enabling compliance tracking, document control, and automated workflows for regulated life science and healthcare environments.
Read MoreOctalsoft WMS
A Warehouse Management System designed to optimize storage, track inventory, and ensure efficient clinical and laboratory material distribution.
Read MoreOctalsoft PPM
A Portfolio and Project Management system that simplifies project planning, resource allocation, and real-time progress tracking across multiple trials.
Read MoreOctalsoft LMS
A Learning Management System delivering training modules, compliance certifications, and skill tracking for clinical and healthcare professionals.
Read MoreOctalsoft BMS
A Biorepository Management System providing traceability, storage optimization, and compliance-driven management of biological samples and inventories.
Read MoreOctalsoft HIMS
A Hospital Information Management System improving patient records, hospital operations, and clinical efficiency through integrated, digital workflows.
Read MoreAdvanced Web Development
Bespoke web applications designed with inclusivity and scalability. Our SaaS and Cloud solutions make your business-focused projects shine, including custom software solutions for pharma organizations.
Read MoreMobile Application Development
Reach new markets with best-in-class mobile apps across iOS, Android, Windows, and more—built with performance and usability at the core. This includes custom pharmaceutical software app development tailored to regulatory standards.
Read MoreLegacy Application Migration
Move beyond outdated systems. Gain agility, reduced time-to-market, and cutting-edge capabilities through seamless legacy application migration, including bespoke software development for pharma businesses transitioning from legacy systems.
Read MoreSoftware Product Development
From inception to go-to-market, we deliver customer-centric products that adapt to evolving needs and challenges, such as custom software development pharma solutions that drive innovation and compliance.
Read MoreEnterprise Application Integration
Streamline your workflows with seamless third-party integrations. Enhance extensibility and flexibility with unified systems that support custom software development pharmaceutical environments.
Read MoreUI/UX Design and Development
Delight your users with intuitive, visually stunning, and user-first designs for smooth navigation and exceptional engagement. Our design approach also supports custom pharmaceutical software app development.
Read MoreOctalsoft:

Seamless Global Reach
Regulatory Adherence
Real-Time Data Access Across Trial Sites
Industries we serve

Pharma & Biotech
Helping companies accelerate the transition from discovery to market with best-in-class clinical trial software solutions.
Read More
CRO
Delivering centralized tools that improve efficiency, transparency, and oversight in clinical research software solutions.
Read More
Hospital
Enabling hospitals and device makers to run reliable and compliant clinical studies.
Read MoreExpert Insights to Drive
Smarter Clinical Trials

ISCR Conference 2026
Join Octalsoft at ISCR 2026, India’s premier clinical research conference shaping the future of clinical trials and life sciences innovation.

SCDM 2025 India Annual Conference
Join us in Hyderabad from December 4-6 for the SCDM 2025 India Conference! This multi-day event has been at the forefront of clinical data excellence,...

DIA 2025 Global Annual Meeting
Octalsoft is attending DIA 2025 Global Annual Meeting from June 15–19 in Washington, DC. This premier event unites regulatory, clinical, safety, and operational leaders to...










 Download
Download