Ensuring Global Compliance With:
Core Capabilities of Octalsoft LMS
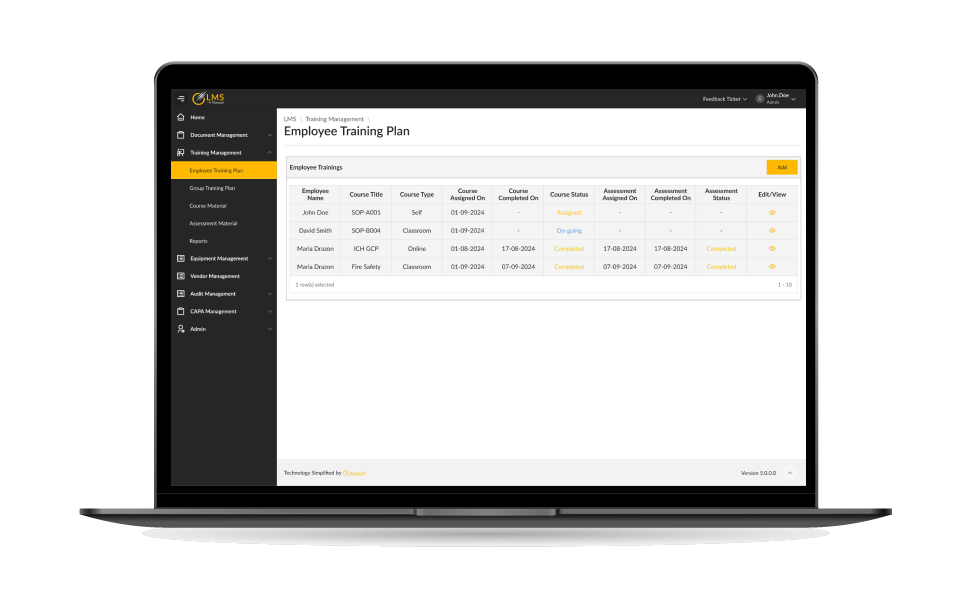
Centralized Learning Hub
- Host, organize, and manage all training programs in one platform.
- Supports e-learning modules, video courses, live sessions, and blended learning.
- Track progress across global teams with ease, including LMS trial programs.
AI-Powered Personalized Learning
- Adaptive learning paths tailored to each user’s role and needs.
- AI-driven recommendations ensure learners engage with the most relevant content.
- Keeps employees and clinical teams continuously upskilled with pharmaceutical LMS features.
Compliance-Ready Training
- Built-in GCP, ICH, HIPAA, and 21 CFR Part 11 compliant workflows.
- Automated audit trails for training completions and certifications.
- Ensure regulatory readiness with proof of training records at any time, supporting the LMS in pharmaceutical industry.
Certification & Competency Tracking
- Create role-based certifications for trial staff, CRO teams, and employees.
- Track skill development and validate competencies across functions.
- Automated re-certification reminders to ensure ongoing compliance in LMS pharma environments.
Seamless Collaboration & Accessibility
- Role-based dashboards for learners, managers, and administrators.
- Mobile-friendly, multilingual access for global teams.
- Integrated discussion forums, quizzes, and assignments, ensuring LMS for pharma users stay engaged.
Analytics & Insights
- Real-time dashboards to track learner engagement and course completion.
- Identify knowledge gaps with AI-driven analytics.
- Generate compliance-ready reports for regulators and auditors—ideal for LMS pharmaceutical workflows.
Resources That Speak
for Octalsoft LMS

eClinical Suite Case Study

Factsheet – Learning Management System (LMS)
Enhance Your LMS with These Powerful Add-ons
Key Benefits for Every Role
Business Leaders
Foster a culture of learning, enhance workforce productivity, and improve retention with OTO LMS capabilities.Clinical Trial Managers
Ensure site staff, coordinators, and investigators remain compliant and trained using a modern pharma LMS.Employees & Site Teams
Access easy-to-use learning modules that make training engaging and efficient.Compliance Officers
Maintain audit-ready records of training completions and certifications across LMS laboratoria and trial systems.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download