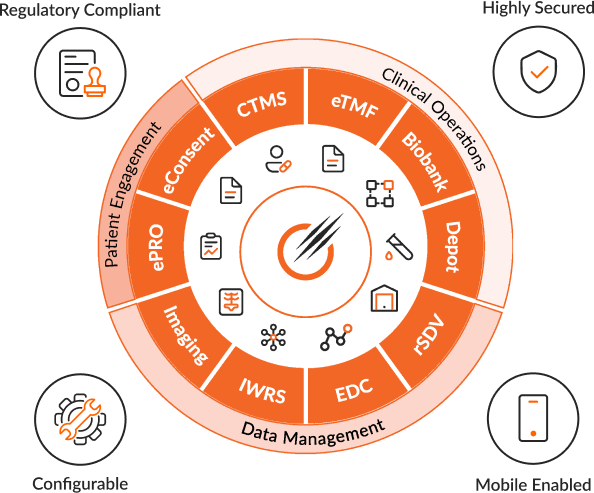
Ensuring Global Compliance With:
Core Capabilities of Octalsoft QMS
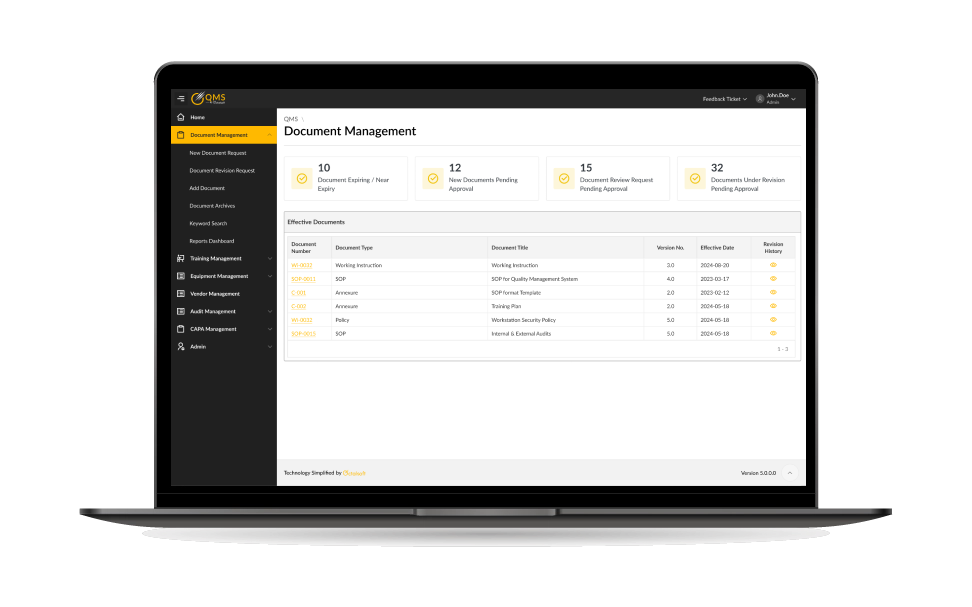
Document & SOP Management
- Centralized repository for policies, SOPs, and controlled documents.
- Automated version control and approval workflows.
- Ensures audit-ready documentation at all times with a robust quality management system for clinical trials.
CAPA (Corrective & Preventive Actions)
- Track and manage CAPA across teams and functions.
- Automated workflows with real-time status tracking.
- Ensures accountability and resolution within defined timelines, strengthening clinical trial quality management.
Regulatory & GxP Compliance
- Supports FDA 21 CFR Part 11, GCP, GMP, ISO, and GDPR compliance.
- Maintains complete audit trails with electronic signatures.
- Ensures readiness for sponsor, CRO, and regulatory audits with a trusted clinical QMS.
Risk & Change Management
- AI-powered risk assessment for processes and documentation.
- Change control workflows with impact analysis.
- Proactive alerts for deviation handling and escalation—ideal for quality management system in biotechnology industry.
Training & Competency Management
- Assign, track, and evaluate staff training requirements.
- Ensures team compliance with GCP, GMP, and SOP-specific protocols.
- Integration with Octalsoft HRMS for unified staff training records in a single clinical quality management system.
Audit & Inspection Management
- Schedule and track internal, external, and regulatory audits.
- Generate audit-ready reports with full traceability.
- Facilitates seamless communication with inspectors and stakeholders, boosting quality management systems for clinical trials.
Resources That Speak
for Octalsoft QMS

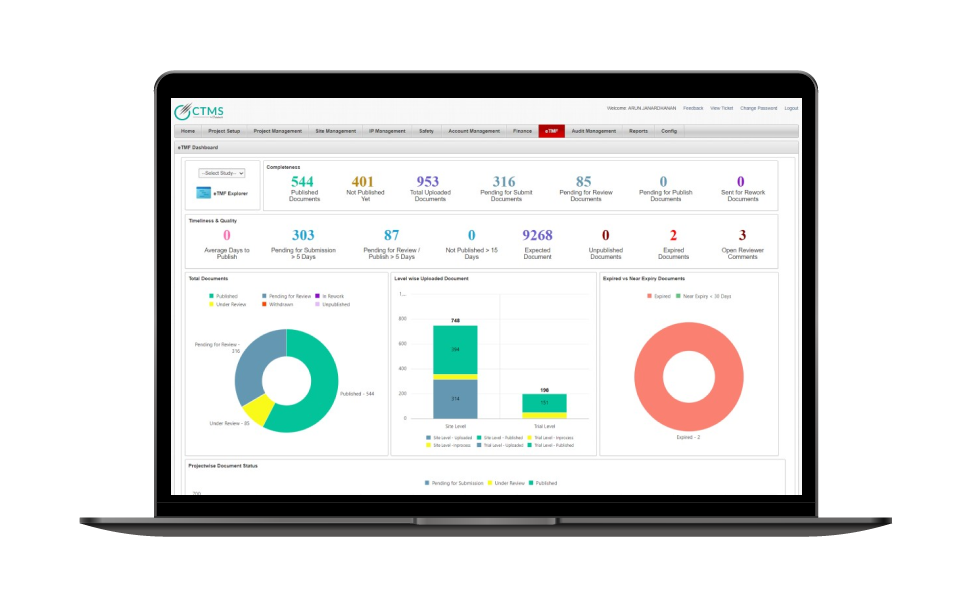
eClinical Suite Case Study

Factsheet – Quality Management System (QMS)
Enhance Your QMS
with These Powerful Add-ons
Octalsoft HRMS
Integrate training records and certifications directly with clinical QMS compliance needs.
Octalsoft eTMF
Align QMS records with trial master files for qms clinical trials inspection readiness.
Key Benefits for Every Role
Business Leaders
Strengthen governance, minimize operational risk, and ensure global quality standards across teams with a unified pharma QMS solution.Clinical Operations
Align trial processes with GCP and sponsor expectations, reducing delays and inspection risks with integrated quality management in clinical trials.Quality Teams
Automate quality workflows, track CAPAs, and maintain oversight with centralized dashboards powered by a modern clinical quality management system.Employees
Easily access SOPs, complete assigned trainings, and ensure compliance without complexity using QMS for clinical trials.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download