Ensuring Global Compliance With:
Core Capabilities of Octalsoft EDC
Intuitive Data Capture Software
- User-friendly interface streamlines data entry for site staff.
- Real-time edit checks minimize errors at the point of capture.
- Configurable eCRFs adapt to diverse clinical research protocols.
- Supports multilingual, multi-site, and global trials.
- Built for both early and late phase EDC requirements.
Rapid Study Setup & Database Build
- Drag-and-drop CRF design tools accelerate trial startup.
- Automated compliance checks during study setup.
- Reusable libraries of forms and edit checks save time across studies.
- Faster go-live with accuracy, scalability, and regulatory confidence.
Real-Time Data Validation & Cleaning
- Built-in edit checks and range validations reduce data queries.
- Automated discrepancy management ensures higher data quality.
- Reconciles data from labs, ePRO, and external systems.
- Fully compliant with global data integrity standards.
Seamless Mid-Study Changes
- Effortlessly manage mid-study amendments with no downtime.
- Version control and audit trails ensure compliance and traceability.
- Adaptive to protocol updates without disrupting ongoing subject data.
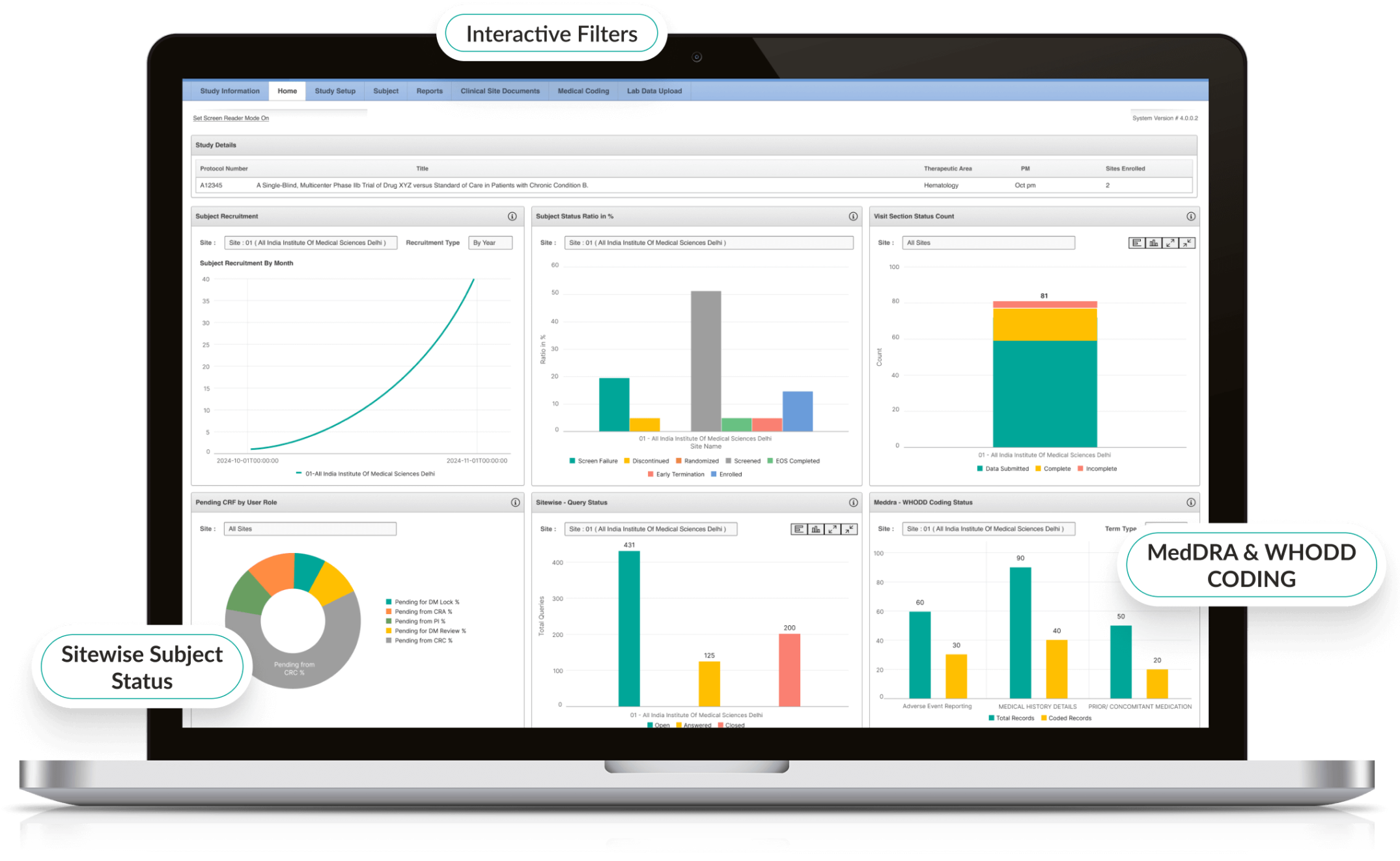
Advanced Reporting & Clinical Trial Dashboards
- Interactive dashboards display enrollment, queries, and site performance.
- Custom reports highlight protocol deviations and emerging trends.
- Visual analytics help sponsors and CROs make informed decisions.
- Export-ready reports for regulators and stakeholders.
Resources That Speak
for Octalsoft EDC

EDC Case Study

Factsheet – Electronic Data Capture (EDC)
Enhance Your EDC
with These Powerful Add-ons
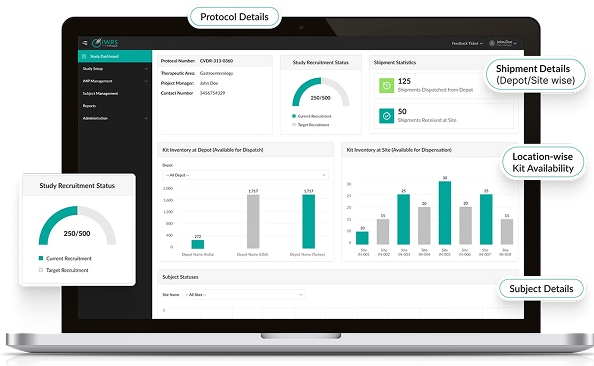
Octalsoft IWRS / RTSM
Connect subject randomization and supply data with your electronic data capture platform for full trial visibility.
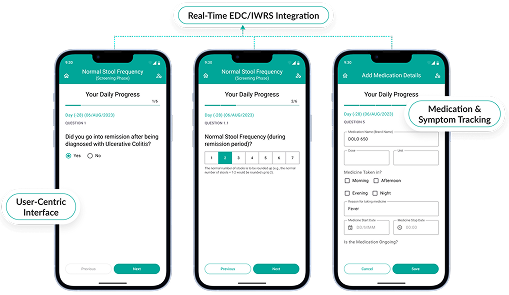
Octalsoft ePRO
Enable patients to submit data remotely, automatically syncing with your EDC system for real-time insights.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Gain real-time oversight of trial data and site performance through centralized monitoring. Accelerate decision-making with accurate, compliant data and built-in quality controls that ensure study integrity.CROs
Manage multiple sponsor studies with standardized, scalable processes. Minimize manual data cleaning and deliver results faster across studies of all sizes — from early-phase to global Phase III trials.Site Teams
Simplify data entry, reduce errors, and save time with intuitive EDC workflows. Access user-friendly tools designed to streamline clinical data capture and ensure accuracy from start to finish.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download