Ensuring Global Compliance With:
Core Capabilities of Octalsoft IWRS / RTSM
Scale with Simplicity
- Simple and effortless UI reduces the learning curve for site staff, including coordinators and investigators.
- Intuitive navigation and clear workflows prevent mistakes in critical tasks like subject enrollment and IP allocation.
- Adaptable to trials of varying complexity (Phase I-IV) and sizes.
- Ensures consistency across multi-center and global clinical trials.
Flexible Randomization Algorithms
- Supports versatile randomization methods: simple, block, stratified, adaptive, and minimization.
- Customizable per study protocol and complexity.
- Handles intricate designs including multi-arm, multi-stage, and response-adaptive trials.
- Ensures accurate and compliant patient assignments.
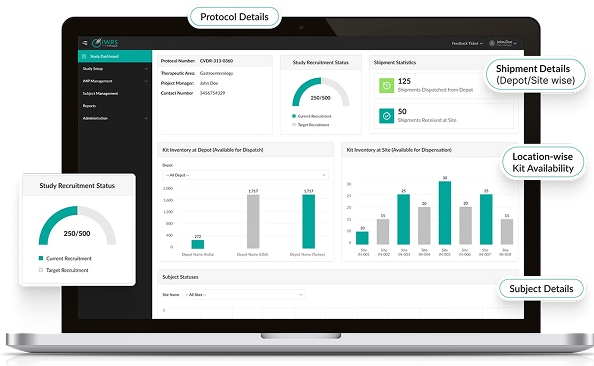
Optimize IP Supply with Predictive Algorithms
- Automates resupply based on real-time consumption and trial milestones.
- Reduces manual effort while improving logistics efficiency.
- Predictive analytics calculate optimal buffer stock and overages.
- Accounts for site variability, trial complexity, and randomization schedules.
Blinding and Allocation Concealment
- Enforces single, double, or triple blinding protocols.
- Uses granular role-based access controls and secure audit trails.
- Protects treatment arm assignments and randomization schedules.
- Maintains trial integrity with built-in concealment logic.
Break the Code Not the Trial
- Replaces manual code envelopes with a secure digital code break system.
- Supports both emergency and non-emergency unblinding.
- Enables real-time authorization workflows for designated users.
- Sends built-in alerts and reports to maintain oversight and compliance.
Track it LIVE-From Start to Finish
- Sends real-time, customizable alerts for events like low stock or temperature excursions.
- Enhances proactive IP replenishment and risk mitigation.
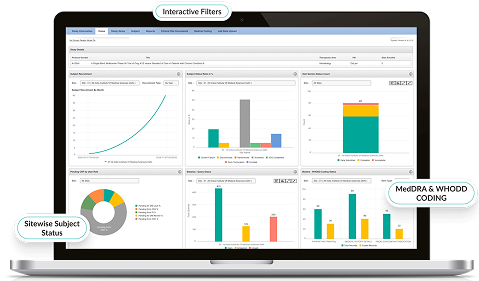
- Generates automated reports on subject progress, IMP usage, and more.
- Empowers data-driven decisions with customized, visual analytics.
Resources That Speak
for Octalsoft IWRS

IWRS + Imaging Case Study

Factsheet – Interactive Web Response System (IWRS)
Enhance Your IWRS
with These Powerful Add-ons
Octalsoft EDC
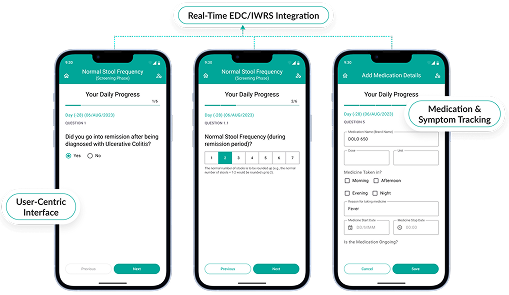
Capture and validate data in real time, seamlessly connected with randomization services.
Octalsoft ePro
Empower patients with remote data entry, integrated into IWRS for holistic trial oversight.
Key Benefits for Every Role in Your Clinical Trial
Sponsors
Streamline trial setup, improve visibility across supply logistics, and make informed decisions with real-time reporting tools tailored to your needs.CROs
Efficiently manage multiple studies across global sites. Simplify protocol-specific configurations and improve collaboration with sponsors and sites.Site Teams
Minimize manual work, eliminate dispensing errors, and manage inventory with ease through our intuitive, user-friendly interface built for site workflows.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.











 Download
Download