Ensuring Global Compliance With:
Core Capabilities of Octalsoft rSDV
AI-Guided Monitoring Workflows
- AI-driven dashboards highlight high-risk sites and subjects.
- Prioritizes source data verification tasks based on risk assessment.
- Guides monitors through streamlined workflows for maximum efficiency.
- Reduces redundancy and ensures accuracy across multiple sites.
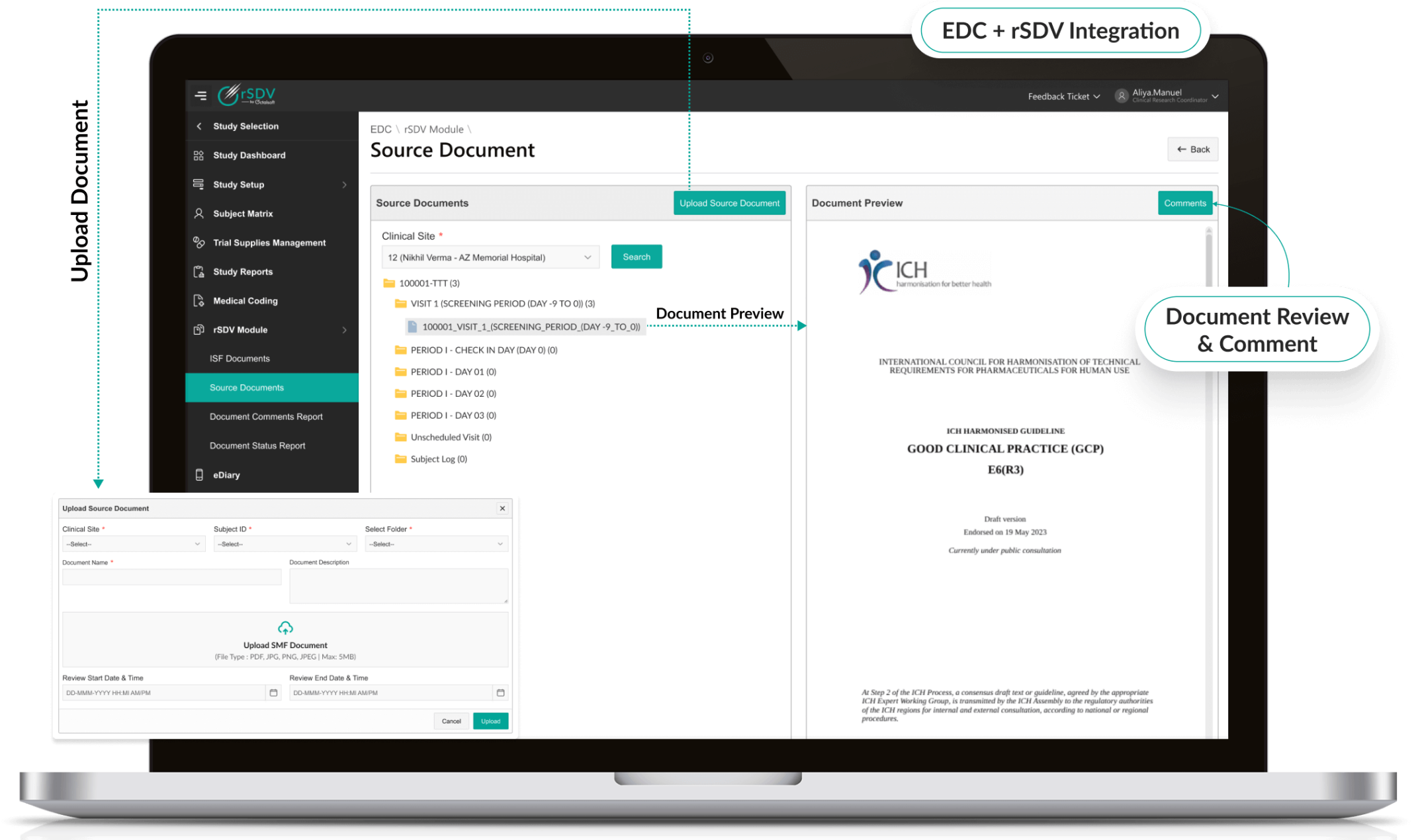
Secure Remote Access
- Provides controlled, encrypted access to source documents.
- Role-based permissions ensure only authorized users view sensitive data.
- AI-powered anomaly detection protects against unauthorized activity.
- Compliant with HIPAA, GDPR, and global privacy regulations.
Real-Time Discrepancy Management
- AI automatically flags inconsistencies between source data and EDC entries.
- Enables rapid query generation and resolution.
- Improves data quality while reducing manual SDV checks.
- Supports reconciliation across labs, imaging, and external systems.
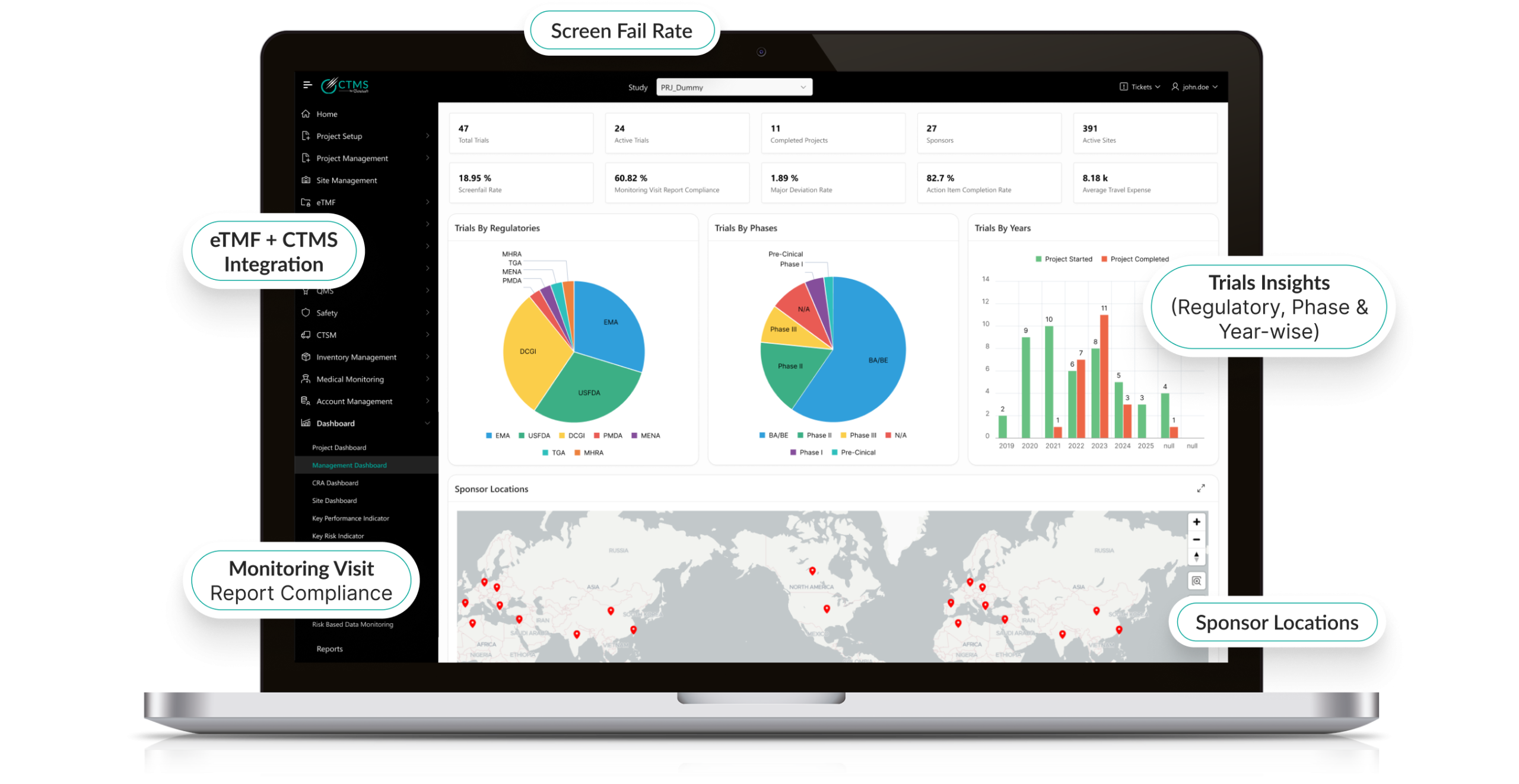
Intelligent Risk-Based Monitoring (RBM)
- Integrates with centralized RBM strategies.
- AI predicts which sites or patients are most likely to deviate from protocol.
- Prioritizes monitoring visits based on data-driven insights.
- Reduces overall monitoring costs while improving compliance.
Seamless Collaboration Tools
- Secure communication channels for CRA, sponsor, and site staff.
- AI organizes and prioritizes issues for resolution.
- Centralized tracking of monitoring notes, actions, and follow-ups.
- Improves transparency and collaboration across stakeholders.
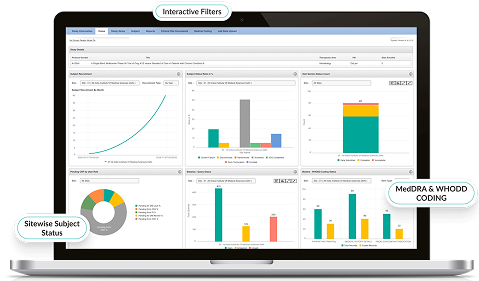
Advanced Analytics & Reporting
- Customizable dashboards visualize site performance, query status, and SDV compliance metrics.
- AI-driven insights predict trends and highlight outliers.
- Automated reporting accelerates decision-making for sponsors and CROs.
- Enables faster trial progress with greater oversight.
Resources That Speak
for Octalsoft rSDV

eClinical Suite Case Study

Factsheet – Remote Source Data Verification (rSDV)
Enhance Your rSDV
with These Powerful Add-ons
Octalsoft EDC
Direct integration with EDC enables real-time source data verification checks and seamless query resolution.
Octalsoft CTMS
Combine monitoring oversight with full operational visibility across trials.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Gain real-time oversight with AI-driven dashboards, reduce monitoring costs, and ensure global compliance.CROs
Scale monitoring operations efficiently across multiple studies. Improve collaboration with sites through centralized, remote source document verification.Site Teams
Reduce disruption from frequent on-site visits. Simplify query resolution with intuitive tools and secure communication channels.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download