Ensuring Global Compliance With:
Core Capabilities of Octalsoft Imaging
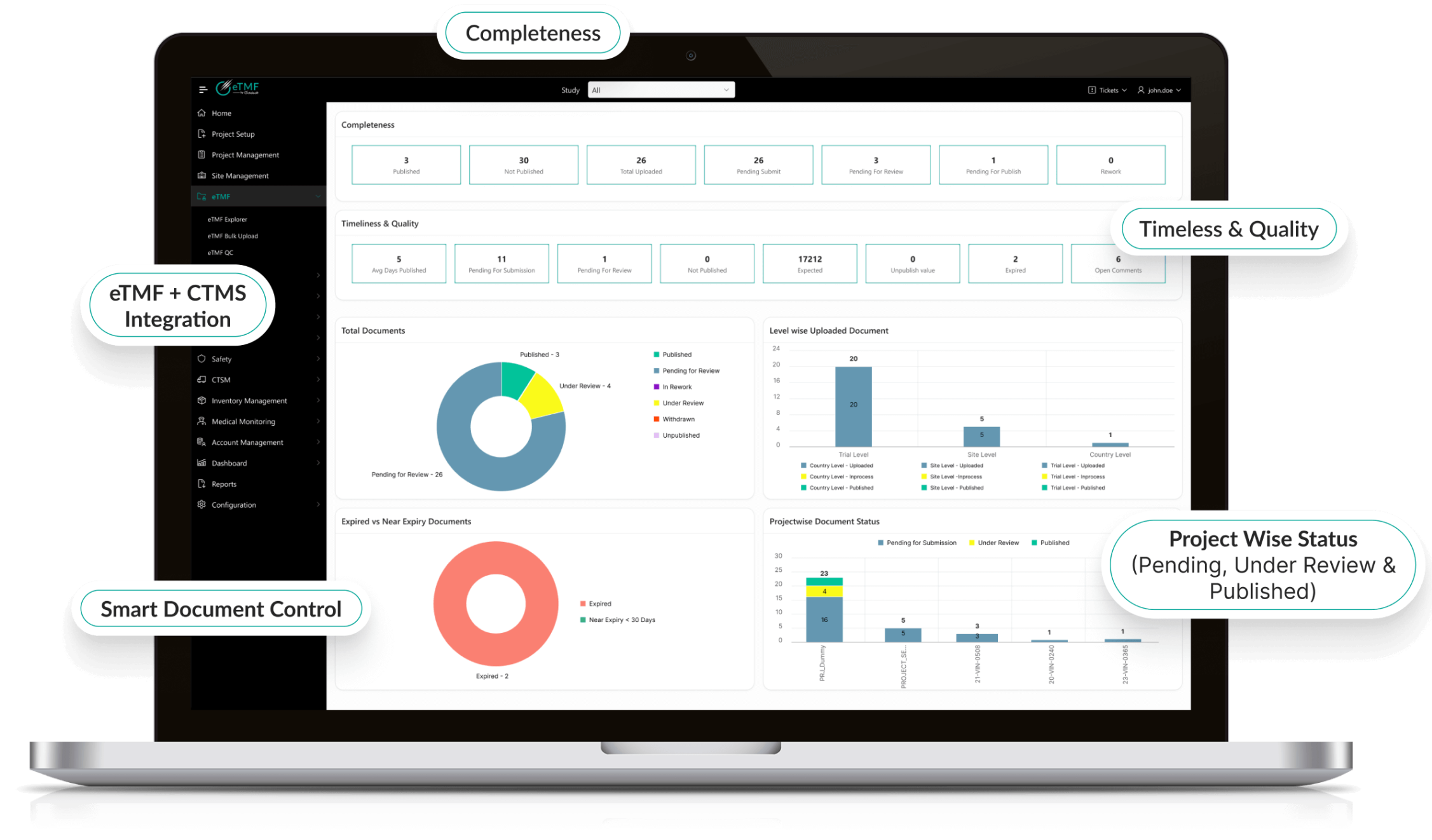
End-to-End Image Management
- Collects, de-identifies, and transfers images from sites to central reviewers.
- Supports multiple modalities (CT, MRI, PET, X-ray, Ultrasound, and more).
- Built-in DICOM compatibility ensures smooth integration with site systems.
- Functions as a complete clinical image management system for global trials.
AI-Powered Image QC
- Automated quality checks detect missing slices, incorrect orientations, or protocol deviations.
- Reduces delays caused by manual verification.
- Ensures images meet study requirements before central review.
- AI-enhanced QC built for clinical trial imaging software standards.
Central Review Workflows
- Facilitates blinded reads with configurable reviewer workflows.
- Supports single, double, or adjudicated review models.
- Built-in audit trails ensure compliance and transparency.
- Optimized for CROs seeking advanced clinical imaging systems.
Seamless Blinding & Masking
- Advanced blinding tools conceal patient identifiers and treatment assignments.
- Role-based access ensures secure, compliant image sharing.
- Preserves trial integrity through controlled unblinding when required.
Real-Time Visibility & Alerts
- Live dashboards track image submissions, QC outcomes, and review status.
- Automated alerts notify stakeholders of delays, missing data, or failed QC.
- Supports proactive issue resolution to avoid bottlenecks.
- Empowers sponsors with clinical data management images in real time.
Regulatory & Compliance Ready
- Designed for HIPAA, GDPR, 21 CFR Part 11, and GCP compliance.
- Inspection-ready audit trails and metadata.
- Ensures global data protection and regulatory alignment.
- Trusted by leading imaging vendors for clinical trials worldwide.
Resources That Speak for
Octalsoft Imaging

IWRS + Imaging Case Study

Factsheet – Imaging
Enhance Your Imaging with
These Powerful Add-ons
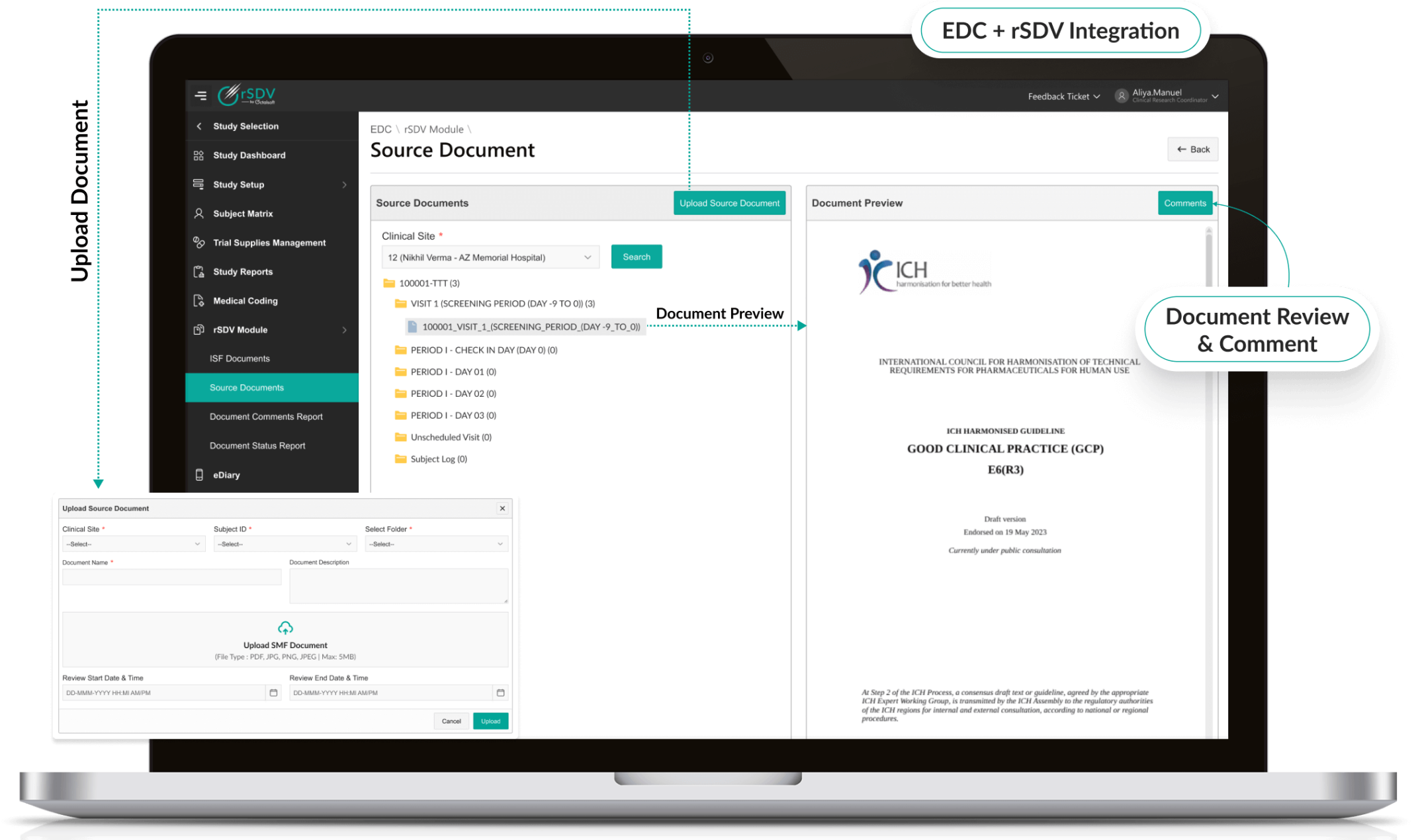
Octalsoft eTMF
Keep all imaging data and metadata inspection-ready in a compliant repository.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Accelerate imaging-based endpoints with centralized, AI-enhanced quality control and review. Gain complete visibility into imaging data flow across all global sites for faster, more confident decisions.CROs
Standardize medical imaging processes across multiple trials with unified workflows. Simplify complex operations, reduce turnaround times, and ensure consistent data quality.Sites
Easily upload, manage, and share images through intuitive, guided workflows. Minimize errors and maintain compliance with automated quality and consistency checks.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download