Ensuring Global Compliance With:
Core Capabilities of Octalsoft ePRO
AI-Driven Patient Engagement
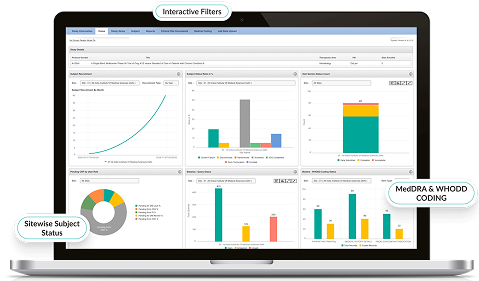
- Customizable dashboards visualize site performance, query status, and SDV compliance metrics.
- AI-driven insights predict trends and highlight outliers.
- Automated reporting accelerates decision-making for sponsors and CROs.
- Enables faster trial progress with greater oversight.
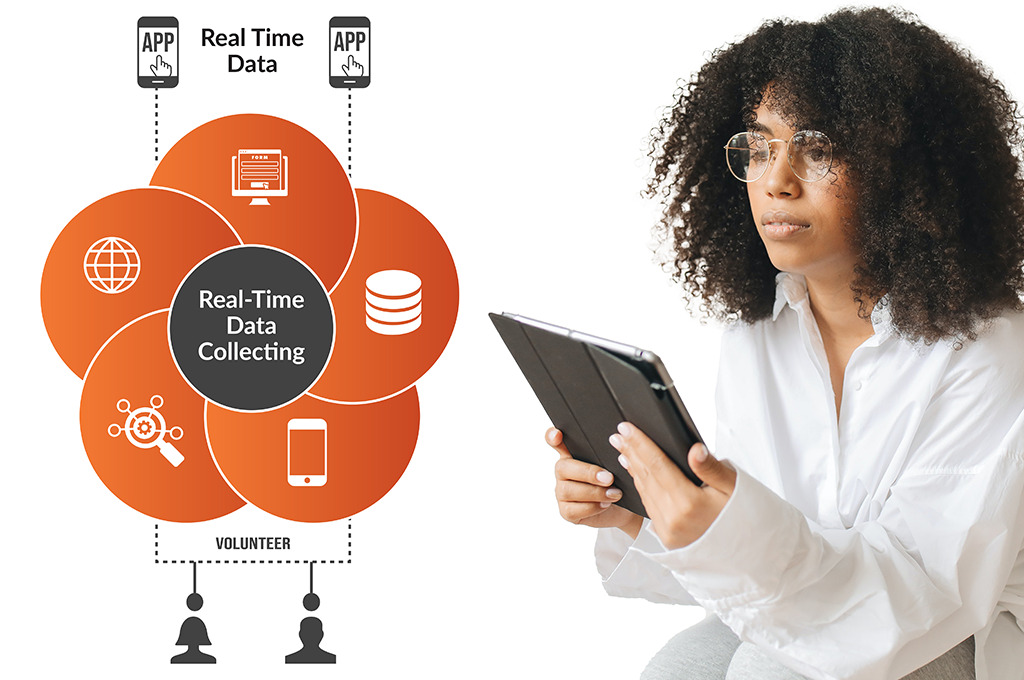
Real-Time Data Capture & Validation
- Secure patient data entry via smartphone, tablet, or web.
- AI-driven edit checks flag incomplete or inconsistent responses.
- Real-time data syncing ensures immediate investigator access.
- Supports multimedia inputs including images, audio, and wearables.
Intelligent Compliance Monitoring
- AI tracks patient adherence and flags potential risks.
- Automated alerts notify sites of missed or delayed entries.
- Predictive analytics forecast patient retention trends.
- Enhances oversight while reducing burden on patients.
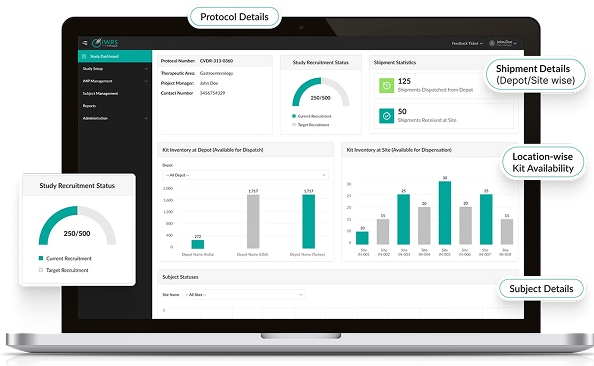
Seamless Workflow Integration
- Native integration with Octalsoft EDC, IWRS, CTMS, and rSDV.
- Direct data reconciliation eliminates transcription errors.
- AI links patient-reported outcomes to clinical endpoints.
- Accelerates study
Advanced Analytics & Insights
- Customizable dashboards visualize patient outcomes and compliance trends.
- AI detects hidden patterns across patient cohorts.
- Export-ready datasets for regulatory submissions.
- Converts raw patient feedback into actionable clinical intelligence.
Enhanced Data Security & Compliance
- End-to-end encryption safeguards patient-reported data across all devices.
- Role-based access control ensures only authorized personnel can view sensitive data.
- Automated audit trails maintain full traceability of all data interactions.
- Fully compliant with global regulations including 21 CFR Part 11, GDPR, and HIPAA.
Resources That Speak
for Octalsoft ePRO

ePRO Integrated IWRS Solution Case Study

Factsheet – Electronic Patient Reported Outcome (ePRO)
Enhance Your ePRO
with These Powerful Add-ons
Octalsoft EDC
Seamlessly integrate ePRO with your study database for real-time data validation and analysis.
Octalsoft IWRS / RTSM
Connect patient-reported outcomes with randomization and supply management workflows.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Unlock deeper insights into patient experiences with unified, real-world data. Reduce compliance risks and accelerate decision-making through centralized oversight. Enhance trial outcomes by integrating patient insights into every stage of the study.CROs
Standardize patient engagement processes across global studies and sponsors. Manage operations through centralized dashboards with predictive analytics that drive proactive performance improvement. Ensure consistency, compliance, and scalability across all patient touchpoints.Site Teams
Reduce administrative burden with automated compliance and adherence tracking. Monitor patient engagement in real time to improve retention and satisfaction. Foster stronger communication and trust with participants throughout the trial journey.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.











 Download
Download