Ensuring Global Compliance With:
Core Capabilities of Octalsoft eConsent
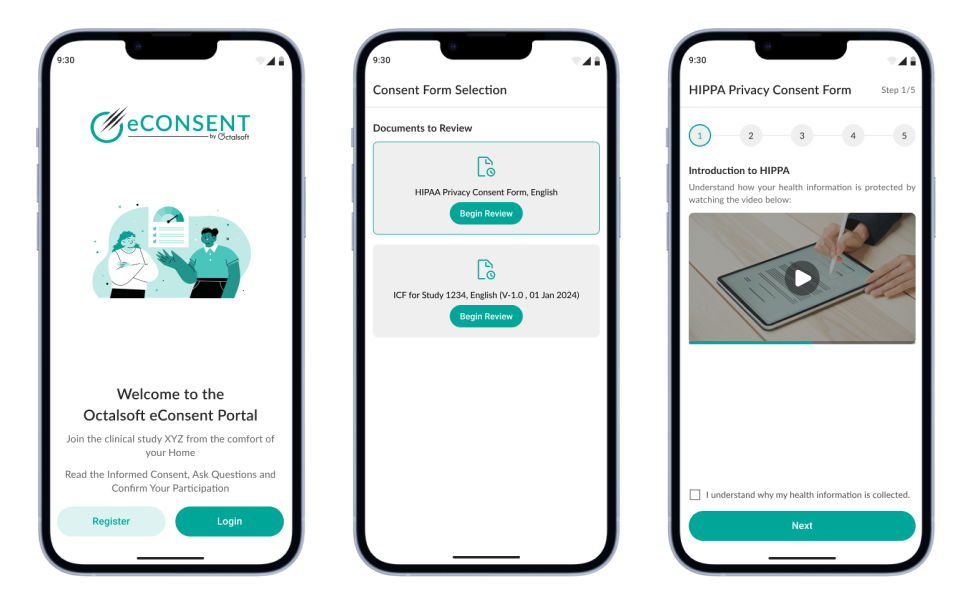
AI-Enhanced Patient Understanding
- Multimedia-rich consent forms (video, audio, graphics) improve comprehension.
- Adaptive learning quizzes verify understanding in eConsent clinical trials.
- Supports multiple languages and accessibility options for global patient reach.
- Ensures truly informed decision-making with patient consent software.
Streamlined Digital Workflows
- Digitizes the complete clinical trial consent documentation software process—from creation to signing and storage.
- AI detects incomplete forms or missing approvals.
- Real-time updates sync across all sites and stakeholders.
- Eliminates paper errors and reduces trial delays.
Secure Identity Verification
- E-signatures with advanced identity authentication.
- AI-powered fraud detection strengthens trust in electronic consent software.
- Fully compliant with FDA 21 CFR Part 11, ICH-GCP, and GDPR.
- Protects patients and sponsors from regulatory risk.
Version Control & Audit Readiness
- Automatic version tracking across sites and protocols.
- AI prevents outdated eConsent forms from circulation.
- Audit-ready logs ensure compliance and traceability.
- Simplifies regulatory inspections with consent application software.
Real-Time Oversight & Analytics
- Centralized clinical trial eConsent dashboard for all sites.
- AI predicts consent bottlenecks before they impact enrollment.
- Generates compliance and enrollment metrics for sponsors and CROs.
- Enables faster trial startup with transparent, data-driven oversight.
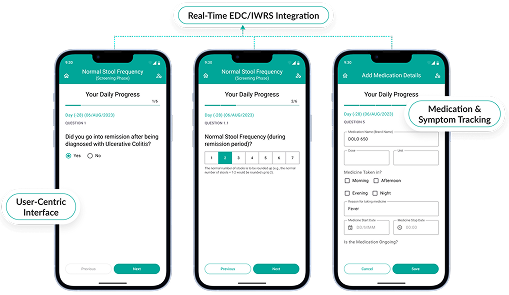
Seamless Integration & Interoperability
- Effortlessly connects with CTMS, EDC, and ePRO systems for unified trial management.
- AI ensures data consistency across platforms, eliminating duplicate entries.
- Supports
Resources That Speak
for Octalsoft eConsent

eClinical Suite Case Study

Factsheet – Electronic Informed Consent Form (eCONSENT)
Enhance Your eConsent
with These Powerful Add-ons
Octalsoft ePRO
Integrate patient-reported outcomes with eConsent clinical trials workflows.
Octalsoft CTMS
Link consent oversight to trial-wide operations via a unified patient consent solution.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Accelerate study startup with streamlined eConsent workflows and centralized oversight. Reduce errors and ensure global compliance through unified monitoring and reporting.CROs
Manage consent efficiently across multiple studies and protocols with scalable eConsent solutions. Leverage AI-driven analytics for proactive risk prediction and performance insights.Sites
Simplify patient interactions, reduce administrative tasks, and enhance trial oversight. Ensure every participant is properly informed with intuitive, compliant eConsent tools.Patient
Access clear, multilingual, and multimedia consent forms designed for understanding and ease. Make confident participation decisions through an intuitive, patient-centered eConsent experience.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download