Ensuring Global Compliance With:
Core Capabilities of Octalsoft eTMF
Centralized Trial Documentation
- Cloud-based repository for all trial master file documents.
- AI-powered categorization and indexing for fast, reliable retrieval.
- Pre-mapped to the DIA eTMF Reference Model for standardization.
- Scales effortlessly across Phase I–IV and global, multi-site studies.
AI-Driven Quality & Completeness Checks
- Automated QC to meet regulatory and protocol standards.
- AI flags missing, incomplete, or duplicate records.
- Risk-based scoring highlights compliance gaps for faster remediation.
- Supports continuous audit and inspection readiness.
Intelligent Version Control & Audit Trail
- Secure version management with controlled access.
- AI detects unauthorized edits and inconsistencies.
- Comprehensive audit trail captures every change, approval, and e-signature.
- Fully compliant with 21 CFR Part 11, ICH-GCP, HIPAA, and GDPR.
Seamless Collaboration Across Stakeholders
- Role-based access ensures secure sponsor–CRO–site collaboration.
- Automated workflows streamline document review and approval cycles.
- Real-time notifications keep trial teams aligned.
- Reduces bottlenecks in submissions and monitoring.
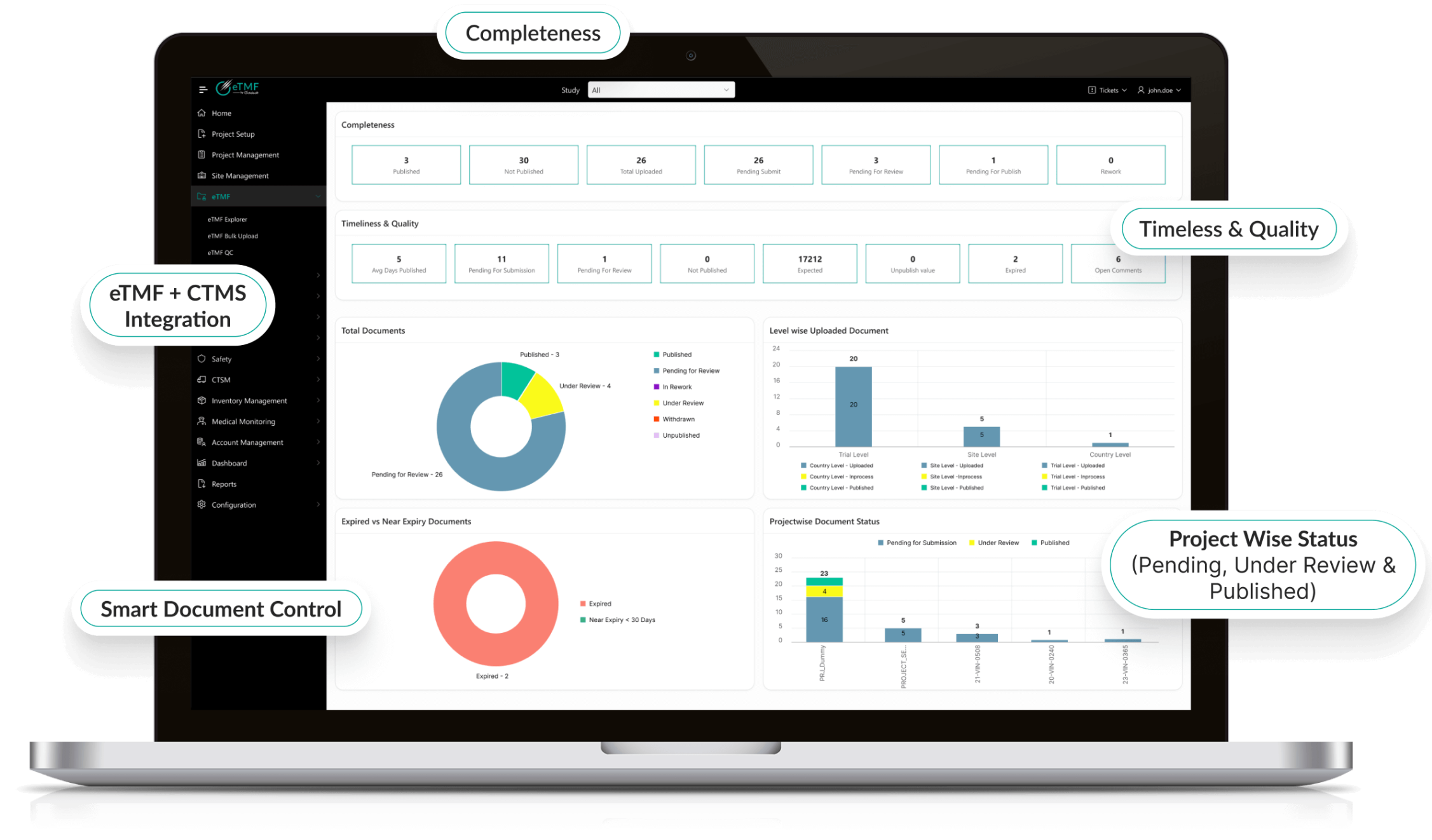
Real-Time Oversight & Analytics
- Custom dashboards track trial progress and TMF health metrics.
- AI-driven analytics predict compliance risks proactively.
- Automated reporting accelerates decision-making and submissions.
- Supports both sponsors and CROs with transparent oversight.
Resources That Speak
for Octalsoft eTMF

eClinical Suite Case Study

Factsheet – Electronic Trial Master File (eTMF)
Enhance Your eTMF
with These Powerful Add-ons
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Ensure global compliance with real-time visibility and centralized document oversight. Enhance TMF quality and maintain continuous inspection readiness across all studies.CROs
Standardize documentation processes across multiple sponsors for improved efficiency. Scale effortlessly while minimizing administrative workload and compliance risks.Site Teams
Simplify document management and stay aligned with sponsor requirements. Reduce manual effort and maintain audit-ready documentation without added burden.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.










 Download
Download



