Ensuring Global Compliance With:
Core Capabilities of Octalsoft CTMS
Centralized Trial Oversight
- Gain a 360° view of all study activities in real time with one unified clinical trial management system software.
- Consolidate trial timelines, milestones, and deliverables into a single dashboard.
- Enable proactive risk management through real-time alerts and analytics.
- Ensure operational consistency across global and multi-site clinical trials software deployments.
Smarter Study Planning & Setup
- Accelerate trial startup with configurable workflows in a powerful CTMS clinical trial platform.
- Digitize protocol design, site selection, and budget forecasting.
- Simplify contract tracking, investigator payments, and resource allocation.
- Reduce manual errors with automated checks and validations.
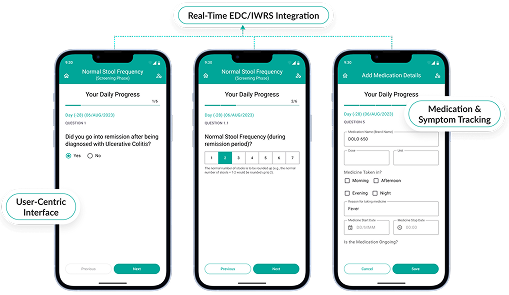
Site & Subject Management Made Simple
- Maintain detailed site profiles, staff credentials, and training records in one clinical research management system.
- Streamline communication between sponsors, CROs, and sites.
- Track subject enrollment, visit schedules, and retention metrics effortlessly.
- Enable compliance with centralized document management.
Financial Transparency & Control
- Automate budgeting, forecasting, and payment schedules within your clinical research software.
- Track investigator grants, reimbursements, and site expenses with accuracy.
- Enable financial reconciliation across multiple geographies and currencies.
- Ensure audit readiness with clear financial reporting trails.
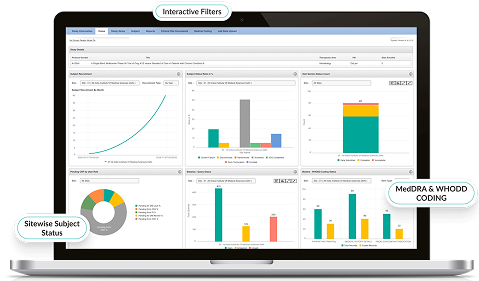
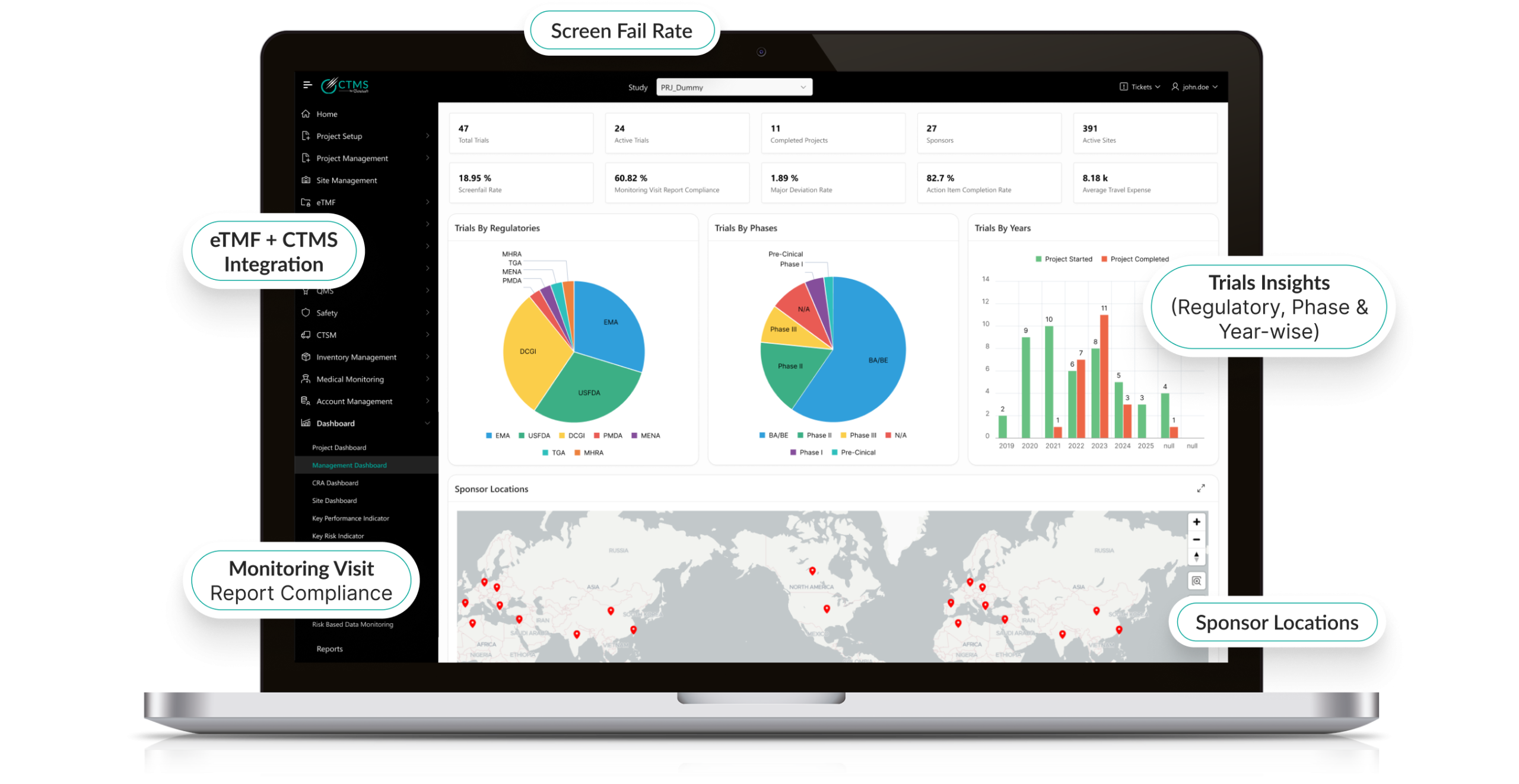
Real-Time Reporting & Analytics
- Generate customizable dashboards for trial progress and KPIs.
- Enable data-driven decision-making with visual analytics.
- Track recruitment, protocol deviations, and monitoring activity live.
- Enhance sponsor-CRO-site collaboration with secure shared insights.
Resources That Speak
for Octalsoft CTMS
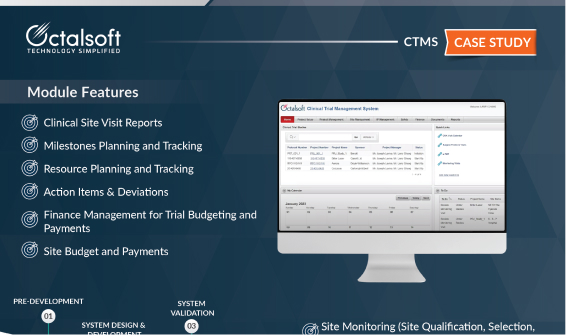
CTMS Case Study

Factsheet – Clinical Trial Management System (CTMS)
Enhance Your CTMS
with These Powerful Add-ons
Octalsoft EDC
Capture, clean, and manage clinical data with ease through a seamlessly integrated clinical trial software solution.
Octalsoft ePRO
Empower patients with intuitive, remote data capture that integrates directly into your CTMS clinical trials workflows.
Key Benefits for Every Role
in Your Clinical Trial
Sponsors
Improve oversight, optimize timelines, and strengthen collaboration across stakeholders with centralized trial intelligence powered by our clinical trial software solutions.CROs
Manage multiple sponsor studies with consistency using CTMS systems for clinical trials. Boost operational efficiency and scale seamlessly across regions and protocols.Site Teams
Reduce administrative burden with easy-to-use tools for visit tracking, document management, and payments—freeing more time for patient care through an advanced clinical research management software.Why Choose Octalsoft?
Domain Expertise
With decades of combined experience in clinical research technology, our team understands the needs of CROs, Sponsors, and Sites.
All-in-One Platform
From planning and operations to data capture and analytics - we deliver integrated platforms for every stage of the trial lifecycle.
Regulatory Ready
Our systems are designed to meet global regulatory standards such as 21 CFR Part 11, GCP, and GDPR, ensuring compliance at every level.
24/7 Support
We offer personalized onboarding, 24/7 support, and ongoing optimization services to ensure long-term success for your studies.











 Download
Download