Product Features
Configurable TMF
Access a flexible wizard-driven process for setting up eTMF – table of contents (TOI), document templates, metadata, and other parameters, allows the user to accurately customize the TMF filing plan with study-specific documents. The plan once configured can be further saved as a reusable template.

TMF Reference Model
The TMF reference model provides standardized taxonomy and outlines a reference definition of TMF content using industry-defined nomenclature. Adherence to the TMF reference model brings order, structure, and lineage to your eTMF – helping you segment and organize data efficiently.

Optimized Search and Retrieval
The Octalsoft eTMF solution leverages the metadata related to your document to help locate the relevant document in a search, even find the content within the artifact. Our experienced team helps you to associate custom metadata fields to fit with your process and study parameters.

Optimized Search and Retrieval
Octalsoft eTMF allows you to define user roles and customize them to your specific process. Each user will have just enough access to the data/document that is required by him to perform his delegated function. Ours is a solution with granular permissions will allow you to set access levels for various functions.

Customizable User Roles
Octalsoft eTMF provides multi-layered security across databases and applications to maintain the integrity and confidentiality of the data. It implements data encryption, authorized access controls, usage analysis, log history, and conditional auditing to meet compliance requirements.

Highly Secured
Octalsoft eTMF software provides timely and relevant alerts and notifications to users to action or complete tasks within the system. Having a contextual, rule-based notification system, based on user roles, significantly reduces the time which is otherwise required for cross-functional communication.
Achieve Standardization
- Standardize trial master file content and processes across all study phases and across multiple studies
- One unified database to store, organize, and distribute your TMF documents
- Integrated workflow, email notifications, and alerts help you automate and streamline processes.

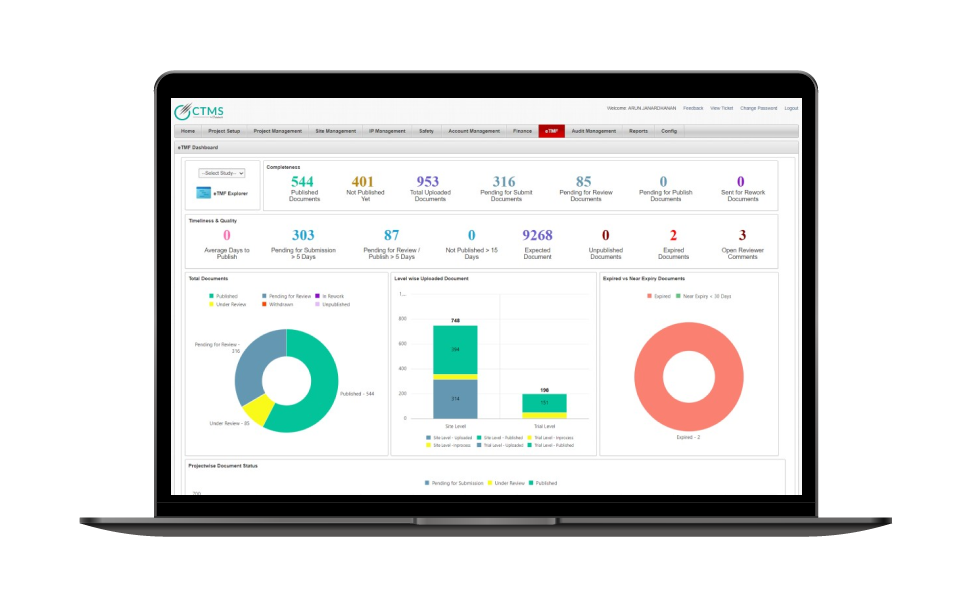
Improve Compliance and reporting
- Real-time insight into the status of the filed documents helps you identify missing and overdue documents and take steps for appropriate action
- Interactive reporting allows users to modify their data layouts without any IT assistance. Each end-user can rearrange a report’s data and save multiple layouts for future reference
- With the help of dynamic reporting tools and business intelligence, you can proactively track the completeness of your TMF in real time.

Our Vetted Experience
Downloads
Related Solutions

CTMS
Maintain a centralized, relevant, and most up to date study and operational database; thus providing users with total control, while complying with all regulations.

EDC
Accelerate the speed of your clinical trial by reducing deployment time, capturing clean data quicker, timely study close-out and early data lock efficiently.

IWRS
Effectively configure subject enrolment and randomization process and also manage global IP supply chains, using an intuitive web-browser interface.